Matching (Value 15)
|
|
|
Match each item with the correct statement below. a. | electronegativity | f. | periodic law | b. | ionization energy | g. | cation | c. | atomic
radius | h. | period | d. | metal | i. | group | e. | transition
metal | j. | electrons |
|
|
|
1.
|
type of element characterized by the presence of electrons in the d
orbital
|
|
|
2.
|
horizontal row in the periodic table
|
|
|
3.
|
type of ion formed by Group 2A elements
|
|
|
4.
|
A repetition of properties occurs when elements are arranged in order of
increasing atomic number.
|
|
|
5.
|
ability of an atom to attract electrons when the atom is in a compound
|
|
|
6.
|
energy required to remove an electron from an atom
|
|
|
7.
|
vertical column in the periodic table
|
|
|
8.
|
type of element that is a good conductor of heat and electric current
|
|
|
Match each item with the correct statement below. a. | monatomic ion | f. | cation | b. | acid | g. | binary compound | c. | base | h. | anion | d. | law of definite proportions | i. | polyatomic ion | e. | law of multiple
proportions |
|
|
|
9.
|
atom or group of atoms having a positive charge
|
|
|
10.
|
compound composed of two different elements
|
|
|
11.
|
atom or group of atoms having a negative charge
|
|
|
12.
|
produces a hydrogen ion when dissolved in water
|
|
|
13.
|
tightly-bound group of atoms that behaves as a unit and carries a net
charge
|
|
|
14.
|
consists of a single atom with a positive or negative charge
|
|
|
15.
|
produces a hydroxide ion when dissolved in water
|
Multiple Choice (Value 47)
Identify the choice that best completes the statement or answers
the question.
|
|
|
16.
|
If E is the symbol for an element, which two of the following symbols represent
isotopes of the same element? a. | 3 and 4 | c. | 1 and 2 | b. | 2 and 3 | d. | 1 and 4 |
|
|
|
17.
|
What do chemical symbols and formulas represent, respectively?
a. | elements and compounds | b. | compounds and mixtures | c. | elements and
ions | d. | atoms and mixtures |
|
|
|
18.
|
Which of the following materials is a substance?
a. | stainless steel | c. | gasoline | b. | silver | d. | air |
|
|
|
19.
|
How are chemical formulas of binary ionic compounds generally written?
a. | subscripts first, then ions | b. | cation on left, anion on
right | c. | Roman numeral first, then anion, then cation | d. | anion on left,
cation on right |
|
|
|
20.
|
Which of the following is true about the composition of ionic compounds?
a. | They are composed of cations only. | b. | They are composed of anions
only. | c. | They are formed from two or more nonmetallic elements. | d. | They are composed of
anions and cations. |
|
|
|
21.
|
Which of the following statements is NOT true about ions?
a. | Charges for ions are written as numbers followed by a plus or minus
sign. | b. | Anions are common among nonmetals. | c. | When a cation forms, more electrons are
transferred to it. | d. | Cations are positively charged
ions. |
|
|
|
22.
|
What is the electron configuration of the oxide ion (O 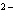 )?
|
|
|
23.
|
Of the elements Fe, Hg, U, and Te, which is a representative element?
|
|
|
24.
|
Atomic size generally ____.
a. | decreases as you move from left to right across a period | b. | remains constant
within a period | c. | decreases as you move from top to bottom within a group | d. | increases as you
move from left to right across a period |
|
|
|
25.
|
A vapor is which state of matter?
a. | liquid | c. | gas | b. | solid | d. | all of the
above |
|
|
|
26.
|
What must be done to be certain that a chemical change has taken place?
a. | Check for the production of bubbles before and after the change. | b. | Demonstrate that a
release of energy occurred after the change. | c. | Demonstrate that energy was absorbed by the
reactants after the change. | d. | Check the composition of the sample before and
after the change. |
|
|
|
27.
|
How many valence electrons are transferred from the calcium atom to iodine in
the formation of the compound calcium iodide?
|
|
|
28.
|
Which subatomic particle plays the greatest part in determining the properties
of an element?
a. | proton | c. | electron | b. | neutron | d. | none of the
above |
|
|
|
29.
|
Which of the following is a chemical property?
a. | freezing point | c. | hardness | b. | ability to react with
oxygen | d. | color |
|
|
|
30.
|
What type of ions have names ending in -ide?
a. | only anions | c. | only metal ions | b. | only cations | d. | only gaseous
ions |
|
|
|
31.
|
Which element, when combined with fluorine, would most likely form an ionic
compound?
a. | carbon | c. | chlorine | b. | lithium | d. | phosphorus |
|
|
|
32.
|
When paper turns yellow-brown upon exposure to sunlight, what type of change is
likely taking place?
a. | neither a physical change nor a chemical change | b. | a chemical
change | c. | both a physical change and a chemical change | d. | a physical
change |
|
|
|
33.
|
In an s orbital, the probability of finding an electron a particular
distance from the nucleus does NOT depend on ____.
a. | a quantum mechanical model | c. | direction with respect to the
nucleus | b. | the Schrodinger equation | d. | the electron energy sublevel |
|
|
|
34.
|
In the chemical reaction in which sucrose is heated and decomposes to form
carbon dioxide and water, which of the following is a reactant?
a. | carbon dioxide | c. | heat | b. | sucrose | d. | water |
|
|
|
35.
|
Which electron configuration of the 4f energy sublevel is the most
stable?
|
|
|
36.
|
Which of the following can be observed only in a microscopic view?
a. | foam insulation | c. | X-ray of a knee joint | b. | structure of a
muscle cell | d. | shape of a
soybean plant |
|
|
|
37.
|
Select the correct symbol for an atom of tritium.
|
|
|
38.
|
Which of the following elements is in the same period as phosphorus?
a. | nitrogen | c. | carbon | b. | oxygen | d. | magnesium |
|
|
|
39.
|
Of the following elements, which one has the smallest first ionization
energy?
a. | boron | c. | carbon | b. | aluminum | d. | silicon |
|
|
|
40.
|
The atomic number of an element is the total number of which particles in the
nucleus?
a. | protons and electrons | c. | neutrons | b. | protons | d. | electrons |
|
|
|
41.
|
When naming a transition metal ion that can have more than one common ionic
charge, the numerical value of the charge is indicated by a ____.
a. | prefix | c. | superscript after the name | b. | Roman numeral
following the name | d. | suffix |
|
|
|
42.
|
What is the formula of the ion formed when phosphorus achieves a noble-gas
electron configuration?
|
|
|
43.
|
Which of the following best describes an example of pure chemistry?
a. | studying chemicals containing carbon | b. | finding an antidote for a new strain of
virus | c. | developing a cure for osteoporosis | d. | testing the effects of lower concentrations of
a drug on humans |
|
|
|
44.
|
Which of the following groupings contains only representative elements?
a. | Cu, Co, Cd | c. | Hg, Cr, Ag | b. | Al, Mg, Li | d. | Ni, Fe, Zn |
|
|
|
45.
|
In which of the following are the symbol and name for the ion given
correctly?
|
|
|
46.
|
Which of the following is NOT an example of chemistry research in the main area
of energy?
a. | producing hook-and-loop tape | b. | developing rechargeable
batteries | c. | determining the usefulness of oil from soybean plants | d. | studying the effects
of insulation |
|
|
|
47.
|
What distinguishes a substance from a mixture?
a. | Substances are compounds, and mixtures are not. | b. | Samples of the same
substance can have different intensive properties. | c. | Mixtures can be separated physically, while
compounds cannot. | d. | Mixtures are groupings of elements, and
compounds are not. |
|
|
|
48.
|
Which of the following does NOT involve a physical change?
a. | decomposing | c. | melting | b. | grinding | d. | mixing |
|
|
|
49.
|
A substance that forms a vapor is generally in what physical state at room
temperature?
a. | solid | c. | liquid or solid | b. | liquid | d. | gas |
|
|
|
50.
|
In which of the following sets is the symbol of the element, the number of
protons, and the number of electrons given correctly?
a. | Cs, 55 protons, 132.9 electrons | c. | Zn, 30 protons, 60
electrons | b. | In, 49 protons, 49 electrons | d. | F, 19 protons, 19
electrons |
|
|
|
51.
|
How many energy sublevels are in the second principal energy level?
|
|
|
52.
|
An -ate or -ite at the end of a compound name usually indicates
that the compound contains ____.
a. | a polyatomic anion | c. | only two elements | b. | fewer electrons than
protons | d. | neutral
molecules |
|
|
|
53.
|
Which of the following is a heterogeneous mixture?
a. | air | c. | oil and vinegar | b. | milk | d. | vinegar in
water |
|
|
|
54.
|
Which of the following is used for chemical symbols today?
a. | numbers | c. | letters | b. | icons | d. | drawings |
|
|
|
55.
|
Which of the following elements has the smallest first ionization energy?
a. | sodium | c. | magnesium | b. | calcium | d. | potassium |
|
|
|
56.
|
Which of the following electron configurations gives the correct arrangement of
the four valence electrons of the carbon atom in the molecule methane (CH 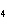 )?
|
|
|
57.
|
Which step in the scientific method requires you to use your senses to obtain
information?
a. | stating a theory | c. | designing an experiment | b. | revising a
hypothesis | d. | making an
observation |
|
|
|
58.
|
Which of the following is NOT a physical change?
a. | mixing two cheeses in a bowl | c. | melting cheese | b. | grating
cheese | d. | fermenting of
cheese |
|
|
|
59.
|
What element in the second period has the largest atomic radius?
a. | lithium | c. | carbon | b. | neon | d. | potassium |
|
|
|
60.
|
What is the relative mass of an electron?
a. | 1/1840 the mass of a hydrogen atom | c. | 1/1840 the mass of a C-12
atom | b. | 1/1840 the mass of a neutron + proton | d. | 1/1840 the mass of an alpha
particle |
|
|
|
61.
|
Which of the following does NOT indicate that a chemical change may have taken
place?
a. | precipitate formation | c. | gas production | b. | energy transfer | d. | fracture
formation |
|
|
|
62.
|
What causes the shielding effect to remain constant across a period?
a. | Electrons are added to different principal energy levels. | b. | Electrons are added
to the same principal energy level. | c. | The atomic radius increases.
| d. | The charge on the nucleus is constant. |
|
Short Answer (Value 11)
|
|
|
63.
|
Give the electron configuration for the lithium ion.
|
|
|
64.
|
What is the electron configuration of oxygen?
|
|
|
65.
|
Write the formula for the compound barium oxide.
|
|
|
66.
|
In which group in the periodic table do the elements have the highest
electronegativity values?
|
|
|
67.
|
List the number of protons, neutrons, and electrons in  C.
|
|
|
68.
|
From which orbital in a lithium atom is an electron transferred to form Li 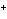 ?
|
|
|
69.
|
What is the electron configuration of sulfur?
|
|
|
70.
|
Give the electron configuration for calcium the ion.
|
|
|
71.
|
What orbital is filled when iodine gains an electron to become a negative
ion?
|
|
|
72.
|
Chlorine has two naturally occurring isotopes, Cl-35 and Cl-37. The atomic mass
of chlorine is 35.45. Which of these two isotopes of chlorine is more abundant?
|
|
|
73.
|
Give the electron configuration for the chloride ion.
|
Numeric Response (Value 10)
|
|
|
74.
|
How many electrons are in the highest occupied energy level of copper?
|
|
|
75.
|
How many electrons are present in the d sublevel of a neutral atom of
nickel?
|
|
|
76.
|
How many electrons are in the highest occupied energy level of a neutral
strontium atom?
|
|
|
77.
|
How many unshared pairs of electrons does the nitrogen atom in ammonia
possess?
|
|
|
78.
|
Use the periodic table to determine the number of electrons in a neutral atom of
lithium.
|
|
|
79.
|
How many electrons are in an atom of gold?
|
|
|
80.
|
How many traditional areas of study can chemistry be divided into?
|
|
|
81.
|
In how many physical states does water commonly exist?
|
|
|
82.
|
How many valence electrons are in bromine?
|
|
|
83.
|
What is the usual charge on an ion from Group 7A?
|
Completion (Value10)
Complete
each statement.
|
|
|
84.
|
1. Write IUPAC names for formulas and formulas
for IUPAC names.
a)
VBr5
b) cobalt(III)nitrate
c) Ni(OH)3
d) bismuth(III)silicate
e) FeO
f)
Fe2O3
g)
Fe(C17H35COO)2
h) tin(II)dichromate
i)
Mn3P4
j) uranium(VI)oxide
|
Complete the below table (Value 21)
|
|
|
85.
|
Complete the follow table:
Isotope Name | Atomic Number | Mass Number | Standard Atomic Notation | # of
Protons | # of Neutrons | Phosphorous-35 | | | 1535P | | | | | | 140 | | 56 | | | | 27 | 60 | | | | | | 92 | | | | 146 | Uranium-235 | | | | | | | | | | | |
|
Essay
|
|
|
86.
|
Formulas and
Constants
Avogadro’s # : 6.02 x10 23
|