Matching
|
|
|
Match each item with the correct statement below. (Value 10) a. | monatomic ion | f. | cation | b. | acid | g. | binary compound | c. | base | h. | anion | d. | law of definite proportions | i. | polyatomic ion | e. | law of multiple
proportions |
|
|
|
1.
|
tightly-bound group of atoms that behaves as a unit and carries a net
charge
|
|
|
2.
|
atom or group of atoms having a negative charge
|
|
|
3.
|
In any chemical compound, the masses of elements are always in the same
proportion by mass.
|
|
|
4.
|
produces a hydrogen ion when dissolved in water
|
|
|
5.
|
atom or group of atoms having a positive charge
|
|
|
6.
|
consists of a single atom with a positive or negative charge
|
|
|
7.
|
compound composed of two different elements
|
|
|
8.
|
produces a hydroxide ion when dissolved in water
|
|
|
9.
|
when two elements form more than one compound, the masses of one element that
combine with the same mass of the other element are in the ratio of small, whole numbers
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.(Value 11)
|
|
|
10.
|
When dissolved in water, acids produce ____.
a. | polyatomic ions | c. | negative ions | b. | hydrogen ions | d. | oxide ions |
|
|
|
11.
|
What is the name of H 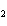 SO 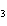 ? a. | sulfuric acid | c. | hydrosulfuric acid | b. | sulfurous acid | d. | hyposulfuric
acid |
|
|
|
12.
|
How are bases named?
a. | like polyatomic ions | c. | like ionic compounds | b. | like monatomic elements | d. | like molecular
compounds |
|
|
|
13.
|
What is the formula for hydrosulfuric acid?
|
|
|
14.
|
Suppose you encounter a chemical formula with H as the cation. What do you know
about this compound immediately?
a. | It is a base. | c. | It has a 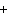 1 charge. 1 charge. | b. | It is an
acid. | d. | It is a polyatomic
ionic compound. |
|
|
|
15.
|
In any chemical compound, the elements are always combined in the same
proportion by ____.
a. | charge | c. | mass | b. | density | d. | volume |
|
|
|
16.
|
What is the formula for sulfurous acid?
|
|
|
17.
|
Which of the following shows both the correct formula and correct name of an
acid?
a. | H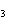 PO PO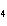 , phosphoric acid , phosphoric acid | c. | HI, iodic
acid | b. | HNO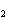 , hydronitrous acid , hydronitrous acid | d. | HClO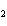 , chloric
acid , chloric
acid |
|
|
|
18.
|
What is the formula for phosphoric acid?
|
|
|
19.
|
When naming acids, the prefix hydro- is used when the name of the acid
anion ends in ____.
|
|
|
20.
|
Which of the following pairs of substances best illustrates the law of multiple
proportions?
|
|
|
21.
|
When the name of an anion that is part of an acid ends in -ite, the acid
name includes the suffix ____.
|