Matching (Value 13)
|
|
|
Match each item with the correct statement below. a. | coordinate covalent bond | d. | single covalent
bond | b. | double covalent bond | e. | polar bond | c. | structural formula | f. | hydrogen bond |
|
|
|
1.
|
a depiction of the arrangement of atoms in molecules and polyatomic
ions
|
|
|
2.
|
a covalent bond in which only one pair of electrons is shared
|
|
|
3.
|
a covalent bond in which two pairs of electrons are shared
|
|
|
4.
|
a covalent bond in which the shared electron pair comes from only one of the
atoms
|
|
|
5.
|
a covalent bond between two atoms of significantly different
electronegativities
|
|
|
6.
|
a type of bond that is very important in determining the properties of water
and of important biological molecules such as proteins and DNA
|
|
|
Match each item with the correct statement below. a. | network solid | e. | tetrahedral angle | b. | bonding orbital | f. | VSEPR theory | c. | dipole
interaction | g. | sigma
bond | d. | bond dissociation energy |
|
|
|
7.
|
energy needed to break a single bond between two covalently bonded
atoms
|
|
|
8.
|
symmetrical bond along the axis between the two nuclei
|
|
|
9.
|
molecular orbital that can be occupied by two electrons of a covalent
bond
|
|
|
10.
|
109.5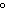
|
|
|
11.
|
shapes adjust so valence-electron pairs are as far apart as possible
|
|
|
12.
|
attraction between polar molecules
|
|
|
13.
|
crystal in which all the atoms are covalently bonded to each other
|
Multiple Choice (Value 10)
Identify the choice that best completes the statement or answers
the question.
|
|
|
14.
|
Molecular orbital theory is based upon which of the following models of the
atom?
a. | classical mechanical model | c. | quantum mechanical
model | b. | Bohr model | d. | Democritus’s model |
|
|
|
15.
|
How is a pair of molecular orbitals formed?
a. | by the splitting of a single atomic orbital | b. | by the reproduction
of a single atomic orbital | c. | by the overlap of two atomic orbitals from the
same atom | d. | by the overlap of two atomic orbitals from different
atoms |
|
|
|
16.
|
The side-by-side overlap of p orbitals produces what kind of bond?
a. | alpha bond | c. | pi bond | b. | beta bond | d. | sigma bond |
|
|
|
17.
|
Which of the following bond types is normally the weakest?
a. | sigma bond formed by the overlap of two s orbitals | b. | sigma bond formed by
the overlap of two p orbitals | c. | sigma bond formed by the overlap of one s
and one p orbital | d. | pi bond formed by the overlap of two p
orbitals |
|
|
|
18.
|
Which of the following theories provides information concerning both molecular
shape and molecular bonding?
a. | molecular orbital theory | c. | orbital hybridization
theory | b. | VSEPR theory | d. | Bohr atomic theory |
|
|
|
19.
|
Experimental evidence suggests that the H—C—H bond angles in ethene,
C 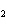 H 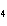 , are ____.
|
|
|
20.
|
How many pi bonds are formed when sp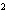 hybridization
occurs in ethene, C 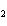 H 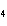 ?
|
|
|
21.
|
Which of the following covalent bonds is the most polar?
|
|
|
22.
|
What is thought to cause the dispersion forces?
a. | attraction between ions | c. | sharing of electron
pairs | b. | motion of electrons | d. | differences in electronegativity |
|
|
|
23.
|
What causes hydrogen bonding?
a. | attraction between ions | b. | motion of electrons | c. | sharing of electron
pairs | d. | bonding of a covalently bonded hydrogen atom with an unshared electron
pair |
|
Numeric Response (Value 1)
|
|
|
24.
|
What is the bond angle in a water molecule?
|
Essay (Value 10)
|
|
|
25.
|
What is bond dissociation energy, and how does it affect carbon
compounds?
|
|
|
26.
|
Indicate how bonding is explained in terms of molecular orbitals.
|
|
|
27.
|
Explain what is meant by VSEPR theory. Give an example of how VSEPR theory can
be applied to predict the shape of a molecule.
|
|
|
28.
|
What determines the degree of polarity in a bond? Distinguish between nonpolar
covalent, polar covalent, and ionic bonds in terms of relative polarity.
|
|
|
29.
|
What are dispersion forces? How is the strength of dispersion forces related to
the number of electrons in a molecule? Give an example of molecules that are attracted to each other
by dispersion forces.
|