Multiple Choice (Value 10)
Identify the choice that best completes the statement or answers
the question.
|
|
|
1.
|
Which is a typical characteristic of an ionic compound?
a. | Electron pairs are shared among atoms. | b. | The ionic compound has a low solubility in
water. | c. | The ionic compound is described as a molecule. | d. | The ionic compound
has a high melting point. |
|
|
|
2.
|
What is shown by the structural formula of a molecule or polyatomic ion?
a. | the arrangement of bonded atoms | c. | the number of metallic
bonds | b. | the number of ionic bonds | d. | the shapes of molecular orbitals |
|
|
|
3.
|
Which of these elements does not exist as a diatomic molecule?
|
|
|
4.
|
How do atoms achieve noble-gas electron configurations in single covalent
bonds?
a. | One atom completely loses two electrons to the other atom in the
bond. | b. | Two atoms share two pairs of electrons. | c. | Two atoms share two
electrons. | d. | Two atoms share one electron. |
|
|
|
5.
|
Which noble gas has the same electron configuration as the oxygen in a water
molecule?
a. | helium | c. | argon | b. | neon | d. | xenon |
|
|
|
6.
|
Which of the following electron configurations gives the correct arrangement of
the four valence electrons of the carbon atom in the molecule methane (CH 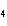 )?
|
|
|
7.
|
A molecule with a single covalent bond is ____.
|
|
|
8.
|
When H 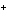 forms a bond with H 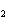 O
to form the hydronium ion H 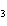 O 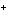 , this bond is called a
coordinate covalent bond because ____. a. | both bonding electrons come from the oxygen atom | b. | it forms an
especially strong bond | c. | the electrons are equally
shared | d. | the oxygen no longer has eight valence electrons |
|
|
|
9.
|
How many valid electron dot formulas—having the same number of electron
pairs for a molecule or ion—can be written when a resonance structure occurs?
a. | 0 | c. | 2 only | b. | 1 only | d. | 2 or more |
|
|
|
10.
|
In which of the following compounds is the octet expanded to include 12
electrons?
|
Numeric Response (Value 5)
|
|
|
11.
|
How many electrons does a nitrogen atom need to gain in order to attain a
noble-gas electron configuration?
|
|
|
12.
|
How many electrons does carbon need to gain in order to obtain a noble-gas
electron configuration?
|
|
|
13.
|
How many electrons are shared in a double covalent bond?
|
|
|
14.
|
How many covalent bonds are in a covalently bonded molecule containing 1
phosphorus atom and 3 chlorine atoms?
|
|
|
15.
|
What is the bond angle in a water molecule?
|
Essay
|
|
|
16.
|
What is bond dissociation energy, and how does it affect carbon
compounds?
|