Matching (Value 5)
|
|
|
Match each item with the correct statement below. a. | halide ion | e. | valence electron | b. | octet rule | f. | coordination number | c. | ionic
bond | g. | metallic
bond | d. | electron dot structure |
|
|
|
1.
|
Atoms react so as to acquire the stable electron structure of a noble
gas.
|
|
|
2.
|
a depiction of valence electrons around the symbol of an element
|
|
|
3.
|
an anion of chlorine or other halogen
|
|
|
4.
|
the force of attraction binding oppositely charged ions together
|
|
|
5.
|
the attraction of valence electrons for metal ions
|
Multiple Choice (Value 10)
Identify the choice that best completes the statement or answers
the question.
|
|
|
6.
|
How many valence electrons are in an atom of phosphorus?
|
|
|
7.
|
How many valence electrons are in an atom of magnesium?
|
|
|
8.
|
What is the formula of the ion formed when potassium achieves noble-gas electron
configuration?
|
|
|
9.
|
Which of the following elements does NOT form an ion with a charge of 1 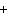 ? a. | fluorine | c. | potassium | b. | hydrogen | d. | sodium |
|
|
|
10.
|
What is the charge on the cation in the ionic compound sodium sulfide?
|
|
|
11.
|
How many valence electrons are transferred from the nitrogen atom to potassium
in the formation of the compound potassium nitride?
|
|
|
12.
|
What is the formula unit of sodium nitride?
|
|
|
13.
|
What is the name of the ionic compound formed from lithium and bromine?
a. | lithium bromine | c. | lithium bromium | b. | lithium bromide | d. | lithium bromate |
|
|
|
14.
|
Which of the following is NOT a characteristic of most ionic compounds?
a. | They are solids. | b. | They have low melting
points. | c. | When melted, they conduct an electric current. | d. | They are composed of
metallic and nonmetallic elements. |
|
|
|
15.
|
Which metallic crystal structure has a coordination number of 8?
a. | body-centered cubic | c. | hexagonal close-packing | b. | face-centered
cubic | d. | tetragonal |
|
Short Answer (Value 4)
|
|
|
16.
|
Write IUPAC names for
formulas and formulas for IUPAC names.
a)
VBr5
b) Ni(OH)3
c) bismuth(III)silicate
______
d) FeO
|
Numeric Response (Value 2)
|
|
|
17.
|
How many valence electrons are in rubidium?
|
|
|
18.
|
What is the charge of a particle having 9 protons and 10 electrons?
|