Matching (Value 5)
|
|
|
Match each item with the correct statement below. a. | Henry's law | d. | supersaturated solution | b. | immiscible | e. | concentration | c. | saturated
solution |
|
|
|
1.
|
describes liquids that are insoluble in one another
|
|
|
2.
|
solution containing maximum amount of solute
|
|
|
3.
|
solution containing more solute than can theoretically dissolve at a given
temperature
|
|
|
4.
|
At a given temperature, the solubility of a gas in a liquid is directly
proportional to the pressure of the gas above the liquid.
|
|
|
5.
|
measure of the amount of solute dissolved in a specified quantity of
solvent
|
Multiple Choice (Value 34)
Identify the choice that best completes the statement or answers
the question.
|
|
|
6.
|
Which of the following usually makes a substance dissolve faster in a
solvent?
a. | agitating the solution | b. | increasing the particle size of the
solute | c. | lowering the temperature | d. | decreasing the number of
particles |
|
|
|
7.
|
What is the maximum amount of KCl that can dissolve in 200 g of water? (The
solubility of KCl is 34 g/100 g H 2O at 20 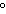 C.) a. | 17 g | c. | 68 g | b. | 34 g | d. | 6800 g |
|
|
|
8.
|
What is the solubility of silver nitrate if only 11.1 g can dissolve in 5.0 g of
water at 20 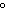 C?
|
|
|
9.
|
Which of the following expressions is generally used for solubility?
a. | grams of solute per 100 grams of solvent | b. | grams of solute per
100 milliliters of solvent | c. | grams of solute per 100 grams of
solution | d. | grams of solute per 100 milliliters of solution |
|
|
|
10.
|
Which of the following pairs of factors affects the solubility of a particular
substance?
a. | temperature and the nature of solute and solvent | b. | temperature and
degree of mixing | c. | particle size and degree of mixing | d. | particle size and
temperature |
|
|
|
11.
|
If a crystal added to an aqueous solution causes many particles to come out of
the solution, the original solution was ____.
a. | unsaturated | c. | an emulsion | b. | saturated | d. | supersaturated |
|
|
|
12.
|
Which of the following substances is less soluble in hot water than in cold
water?
a. | CO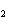 | c. | NaNO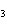 | b. | NaCl | d. | KBr |
|
|
|
13.
|
Which of the following occurs as temperature increases?
a. | Solubility decreases. | c. | Solubility remains the same. | b. | Solubility
increases. | d. | Molarity
doubles. |
|
|
|
14.
|
The solubility of a gas in a liquid is ____.
a. | proportional to the square root of the pressure of the gas above the
liquid | b. | directly proportional to the pressure of the gas above the liquid | c. | inversely
proportional to the pressure of the gas above the liquid | d. | unrelated to the
pressure of the gas above the liquid |
|
|
|
15.
|
If the solubility of a particular solute is 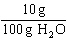 at 20 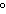 C, which of the
following solution concentrations would represent a supersaturated aqueous solution of that
solute?
|
|
|
16.
|
What happens to the solubility of a gas, in a liquid, if the partial pressure of
the gas above the liquid decreases?
a. | The solubility decreases. | c. | The solubility remains the
same. | b. | The solubility increases. | d. | The solubility cannot be determined. |
|
|
|
17.
|
To increase the solubility of a gas at constant temperature from 1.20 g/L, at
1.4 atm, to 2.3 g/L, the pressure would have to be increased to ____.
a. | 0.37 atm | c. | 1.37 atm | b. | 0.7 atm | d. | 2.7 atm |
|
|
|
18.
|
If the solubility of a gas in water is 4.0 g/L when the pressure of the gas
above the water is 3.0 atm, what is the pressure of the gas above the water when the solubility of
the gas is 1.0 g/L?
a. | 0.75 atm | c. | 4.0 atm | b. | 1.3 atm | d. | 12 atm |
|
|
|
19.
|
What is the molarity of a solution that contains 6 moles of solute in 2 liters
of solution?
|
|
|
20.
|
In which of the following is the solution concentration expressed in terms of
molarity?
|
|
|
21.
|
Which of the following operations yields the number of moles of solute?
a. | molarity 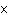 moles of solution moles of solution | c. | molarity 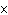 mass of
solution mass of
solution | b. | molarity 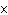 liters of solution liters of solution | d. | moles of solution 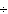 volume of
solution volume of
solution |
|
|
|
22.
|
What is the molarity of a solution containing 7.0 moles of solute in 569 mL of
solution?
|
|
|
23.
|
What is the molarity of 200 mL of solution in which 2.0 moles of sodium bromide
is dissolved?
|
|
|
24.
|
What is the number of moles of solute in 250 mL of a 0.4M
solution?
a. | 0.1 mol | c. | 0.62 mol | b. | 0.16 mol | d. | 1.6 mol |
|
|
|
25.
|
What is the molarity of a solution containing 56 grams of solute in 959 mL of
solution? (molar mass of solute = 26 g/mol)
a. | 1.5M | c. | 2.1M | b. | 2.2M | d. | 0.0022M |
|
|
|
26.
|
What mass of Na 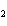 SO 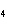 is needed to make 2.5 L of
2.0 M solution? (Na = 23 g; S = 32 g; O = 16 g) a. | 178 g | c. | 356 g | b. | 284 g | d. | 710 g |
|
|
|
27.
|
What does NOT change when a solution is diluted by the addition of
solvent?
a. | volume of solvent | c. | number of moles of solute | b. | mass of
solvent | d. | molarity of
solution |
|
|
|
28.
|
How many mL of a 2.0M NaBr solution are needed to make 200.0 mL of
0.50M NaBr?
a. | 25 mL | c. | 100 mL | b. | 50 mL | d. | 150 mL |
|
|
|
29.
|
The volume of 6.00M HCl needed to make 319 mL of 6.80M HCl is
____.
a. | 0.128 mL | c. | 281 mL | b. | 7.8 mL | d. | 362 mL |
|
|
|
30.
|
If 2.0 mL of 6.0M HCl is used to make a 500.0-mL aqueous solution, what
is the molarity of the dilute solution?
a. | 0.024M | c. | 0.30M | b. | 0.24M | d. | 0.83M |
|
|
|
31.
|
To 225 mL of a 0.80M solution of KI, a student adds enough water to make
1.0 L of a more dilute KI solution. What is the molarity of the new solution?
a. | 180M | c. | 0.35M | b. | 2.8M | d. | 0.18M |
|
|
|
32.
|
If the percent by volume is 2.0% and the volume of solution is 250 mL, what is
the volume of solute in solution?
a. | 0.5 mL | c. | 5.0 mL | b. | 1.25 mL | d. | 12.5 mL |
|
|
|
33.
|
If the percent (mass/mass) for a solute is 4% and the mass of the solution is
200 g, what is the mass of solute in solution?
a. | 8.0 g | c. | 80 g | b. | 50 g | d. | 800 g |
|
|
|
34.
|
The volume of alcohol present in 620 mL of a 40.0% (v/v) solution of alcohol is
____.
a. | 372 mL | c. | 248 mL | b. | 40.0 mL | d. | 580 mL |
|
|
|
35.
|
How many milliliters of alcohol are in 167 mL of an 85.0% (v/v) alcohol
solution?
a. | 252 mL | c. | 145 mL | b. | 228 mL | d. | 142 mL |
|
|
|
36.
|
In which of the following is concentration expressed in percent by
volume?
|
|
|
37.
|
Which of the following is NOT a colligative property of a solution?
a. | boiling point elevation | c. | vapor pressure
lowering | b. | supersaturation | d. | freezing point depression |
|
|
|
38.
|
Colligative properties depend upon the ____.
a. | nature of the solute | c. | number of solute particles in a solution | b. | nature of the
solvent | d. | freezing point of a
solute |
|
|
|
39.
|
A solute depresses the freezing point because the solute ____.
a. | is colder than the solvent | b. | disrupts crystal formation of the
solvent | c. | tends to sink to the bottom of the solution | d. | has bigger molecules
than the solvent |
|
Short Answer (Value 8)
|
|
|
40.
|
How many liters of a 0.30M solution are needed to give 2.7 moles of
solute?
|
|
|
41.
|
What is the molarity of a solution containing 9.0 moles of solute in 2500 mL of
solution?
|
|
|
42.
|
What is the number of moles of solute in 650 mL of a 0.40M
solution?
|
|
|
43.
|
How many liters of a 1.5M solution are required to yield 5.0 grams of
solute? (molar mass of solute = 30.0 g)
|