Matching (Value 10)
|
|
|
Match each item with the correct statement below. a. | solvation | e. | electrolyte | b. | weak electrolyte | f. | colloid | c. | aqueous
solution | g. | surfactant | d. | solvent |
|
|
|
1.
|
interferes with hydrogen bonding between water molecules
|
|
|
2.
|
homogeneous mixture of water and dissolved substances
|
|
|
3.
|
Solute ions or molecules are surrounded by solvent molecules.
|
|
|
4.
|
compound that will conduct current in the liquid state or in aqueous
solution
|
|
|
5.
|
compound that ionizes incompletely in aqueous solution
|
|
|
Match each item with the correct statement below. a. | dispersed phase | e. | Tyndall effect | b. | surface tension | f. | suspension | c. | Brownian
motion | g. | solute | d. | dispersion medium | h. | emulsion |
|
|
|
6.
|
mixture in which particle size averages greater that 1000 nm in
diameter
|
|
|
7.
|
Colloidal particles spread throughout a suspension.
|
|
|
8.
|
phenomenon observed when beam of light passes through a colloid
|
|
|
9.
|
chaotic movement of colloidal particles
|
|
|
10.
|
colloid of a liquid in a liquid
|
Multiple Choice (Value 10)
Identify the choice that best completes the statement or answers
the question.
|
|
|
11.
|
Which atom in a water molecule has the greatest electronegativity?
a. | one of the hydrogen atoms | b. | both hydrogen atoms | c. | the oxygen
atom | d. | There is no difference in the electronegativities of the atoms in a water
molecule. |
|
|
|
12.
|
What is the term for the dissolving medium in a solution?
a. | solvent | c. | solvator | b. | solute | d. | emulsifier |
|
|
|
13.
|
Which of the following substances dissolves most readily in gasoline?
a. | CH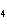 | c. | NH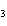 | b. | HCl | d. | NaBr |
|
|
|
14.
|
A solution is a mixture ____.
a. | from which the solute can be filtered | b. | that has the same properties
throughout | c. | that is heterogeneous | d. | in which a solid solute is always dissolved in
a liquid solvent |
|
|
|
15.
|
Why are two nonpolar substances able to dissolve in each other?
a. | They have similar attractive forces in their molecules. | b. | They combine to
produce a polar substance. | c. | There is no attractive force between
them. | d. | Nonpolar substances cannot dissolve in each other. |
|
|
|
16.
|
Which of the following substances dissolves most readily in water?
|
|
|
17.
|
Which of the following compounds is an electrolyte?
a. | rubbing alcohol | c. | carbon tetrachloride | b. | sugar | d. | sodium
hydroxide |
|
|
|
18.
|
Which of the following are weak electrolytes in water?
a. | ionic compounds that partially dissociate in water | b. | ionic compounds that
are soluble | c. | polar compounds that ionize | d. | nonpolar compounds that do not
ionize |
|
|
|
19.
|
Which of the following mixtures is NOT a colloid?
a. | fog | c. | paint | b. | milk | d. | sugar water |
|
|
|
20.
|
Which of these statements is correct?
a. | Particles can be filtered from a suspension. | b. | A solution is
heterogeneous. | c. | A colloidal system does not exhibit the Tyndall effect. | d. | The particles in a
colloidal system are affected by gravity. |
|
Short Answer (Value 4)
|
|
|
21.
|
What is the percentage of water in the hydrate CoCl 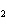 
6 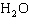 ?
|