Matching (Value 5)
|
|
|
Match each item with the correct statement below. a. | Boyle's law | e. | Gay-Lussac's law | b. | Charles's
law | f. | ideal gas
law | c. | 453oC | g. | 553oC | d. | Graham's
law |
|
|
|
1.
|
For a given mass of gas at constant temperature, the volume of the gas varies
inversely with pressure.
|
|
|
2.
|
The volume of a fixed mass of gas is directly proportional to its Kelvin
temperature, if the pressure is kept constant.
|
|
|
3.
|
The pressure of a gas is directly proportional to its Kelvin temperature if the
volume is kept constant.
|
|
|
4.
|
P ´ V = n ´ R ´ T
|
|
|
5.
|
The Celsius temperature represented by 726 K is
calculated to be
|
Multiple Choice (Value 16)
Identify the choice that best completes the statement or answers
the question.
|
|
|
6.
|
How does the gas propellant move when an aerosol can is used?
a. | from a region of high pressure to a region of lower pressure | b. | from a region of
high pressure to a region of equally high pressure | c. | from a region of low pressure to a region of
higher pressure | d. | from a region of low pressure to a region of equally low
pressure |
|
|
|
7.
|
If a balloon is squeezed, what happens to the pressure of the gas inside the
balloon?
a. | It increases. | b. | It stays the same. | c. | It
decreases. | d. | The pressure depends on the type of gas in the
balloon. |
|
|
|
8.
|
As the temperature of the gas in a balloon decreases, which of the following
occurs?
a. | The volume of the balloon increases. | b. | The average kinetic energy of the gas
decreases. | c. | The gas pressure inside the balloon increases. | d. | all of the
above |
|
|
|
9.
|
What happens to the pressure of a gas inside a container if the temperature of
the gas decreases?
a. | The pressure increases. | c. | The pressure
decreases. | b. | The pressure does not change. | d. | The pressure cannot be
predicted. |
|
|
|
10.
|
Why does air escape from a tire when the tire valve is opened?
a. | The pressure outside the tire is lower than the pressure inside the
tire. | b. | The pressure outside the tire is greater than the pressure inside the
tire. | c. | The temperature is higher outside the tire than inside the tire. | d. | There are more
particles of air outside the tire than inside the tire. |
|
|
|
11.
|
When the Kelvin temperature of an enclosed gas doubles, the particles of the gas
____.
a. | move faster | b. | strike the walls of the container with less
force | c. | decrease in average kinetic energy | d. | decrease in
volume |
|
|
|
12.
|
The volume of a gas is reduced from 4 L to 0.5 L while the temperature is held
constant. How does the gas pressure change?
a. | It increases by a factor of four. | c. | It increases by a factor of
eight. | b. | It decreases by a factor of eight. | d. | It increases by a factor of
two. |
|
|
|
13.
|
If a balloon is heated, what happens to the volume of the air in the balloon if
the pressure is constant?
a. | It increases. | c. | It decreases. | b. | It stays the same. | d. | The change cannot be
predicted. |
|
|
|
14.
|
When the pressure and number of particles of a gas are constant, which of the
following is also constant?
a. | the sum of the volume and temperature in kelvins | b. | the difference of
the volume and temperature in kelvins | c. | the product of the volume and temperature in
kelvins | d. | the ratio of the volume and temperature in kelvins |
|
|
|
15.
|
If a balloon is heated, what happens to the pressure of the air inside the
balloon if the volume remains constant?
a. | It increases. | c. | It decreases. | b. | It stays the same. | d. | The change cannot be
predicted. |
|
|
|
16.
|
What happens when a piston is used to decrease the volume of a contained
gas?
a. | Fewer gas particles exert a force on the piston. | b. | The piston’s
pressure on the gas becomes greater than the pressure exerted by the gas on the
piston. | c. | Gas particles become compressed. | d. | Gas particles leak out of the
container. |
|
|
|
17.
|
What does the ideal gas law allow a scientist to calculate that the other gas
laws do not?
a. | number of moles | c. | volume | b. | pressure | d. | temperature |
|
|
|
18.
|
At a certain temperature and pressure, 0.20 mol of carbon dioxide has a volume
of 3.1 L. A 3.1-L sample of hydrogen at the same temperature and pressure ____.
a. | has the same mass | b. | contains the same number of
atoms | c. | has a higher density | d. | contains the same number of
molecules |
|
|
|
19.
|
How is the ideal gas law usually written?
a. | 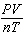 = R = R | c. | PV =
nRT | b. | 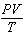 = nR = nR | d. | P = 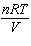 |
|
|
|
20.
|
Which law can be used to calculate the number of moles of a contained
gas?
a. | Boyle’s law | c. | ideal gas law | b. | combined gas law | d. | Charles’s
law |
|
|
|
21.
|
Under what conditions of temperature and pressure is the behavior of real gases
most like that of ideal gases?
a. | low temperature and low pressure | c. | high temperature and low
pressure | b. | low temperature and high pressure | d. | high temperature and high
pressure |
|
Short Answer (Value 6)
|
|
|
22.
|
A gas has a volume of 590 mL at a temperature of –55.0 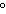 C. What
volume will the gas occupy at 30.0  C?
|
|
|
23.
|
A 10-g mass of krypton occupies 15.0 L at a pressure of 210 kPa. Find the volume
of the krypton when the pressure is increased to 790 kPa.
|
Formulas
|
|
|
24.
|
P1V1 =
P2V2
P1T2 =
P2T1
V1T2 =
V2T1
P1V1T2 =
P2V2T1
PV=nRT
Molarity (M) = moles/liter
|