Matching (Value 10)
|
|
|
Match each item with the correct statement below. a. | Boyle's law | d. | Graham's law | b. | Charles's law | e. | Gay-Lussac's law | c. | Dalton's
law | f. | ideal gas
law |
|
|
|
1.
|
For a given mass of gas at constant temperature, the volume of the gas varies
inversely with pressure.
|
|
|
2.
|
The volume of a fixed mass of gas is directly proportional to its Kelvin
temperature, if the pressure is kept constant.
|
|
|
3.
|
The pressure of a gas is directly proportional to its Kelvin temperature if the
volume is kept constant.
|
|
|
4.
|
P ´ V = n ´ R ´ T
|
|
|
5.
|
At constant volume and temperature, the total pressure exerted by a mixture of
gases is equal to the sum of the partial pressures of the component gases.
|
|
|
6.
|
The rate at which a gas will effuse is inversely proportional to the square
root of the gas’s molar mass.
|
|
|
Match each item with the correct statement below. a. | effusion | c. | diffusion | b. | compressibility | d. | partial pressure
|
|
|
|
7.
|
a measure of how much the volume of matter decreases under pressure
|
|
|
8.
|
the pressure exerted by a gas in a mixture
|
|
|
9.
|
the escape of gas through a small hole in a container
|
|
|
10.
|
tendency of molecules to move to regions of lower concentration
|
Multiple Choice (Value 21)
Identify the letter of the choice that best completes the
statement or answers the question.
|
|
|
11.
|
Why is a gas easier to compress than a liquid or a solid?
a. | Its volume increases more under pressure than an equal volume of liquid
does. | b. | Its volume increases more under pressure than an equal volume of solid
does. | c. | The space between gas particles is much less than the space between liquid or solid
particles. | d. | The volume of a gas’s particles is small compared to the overall volume of the
gas. |
|
|
|
12.
|
How does the gas propellant move when an aerosol can is used?
a. | from a region of high pressure to a region of lower pressure | b. | from a region of
high pressure to a region of equally high pressure | c. | from a region of low pressure to a region of
higher pressure | d. | from a region of low pressure to a region of equally low
pressure |
|
|
|
13.
|
What happens to the temperature of a gas when it is compressed?
a. | The temperature increases. | b. | The temperature does not
change. | c. | The temperature decreases. | d. | The temperature becomes
unpredictable. |
|
|
|
14.
|
As the temperature of the gas in a balloon decreases, which of the following
occurs?
a. | The volume of the balloon increases. | b. | The average kinetic energy of the gas
decreases. | c. | The gas pressure inside the balloon increases. | d. | all of the
above |
|
|
|
15.
|
Which of these changes would NOT cause an increase in the pressure of a
contained gas?
a. | The volume of the container is increased. | b. | More of the gas is
added to the container. | c. | The temperature is
increased. | d. | The average kinetic energy of the gas in increased. |
|
|
|
16.
|
The volume of a gas is doubled while the temperature is held constant. How does
the gas pressure change?
a. | It is reduced by one half. | b. | It does not change. | c. | It is
doubled. | d. | It varies depending on the type of gas. |
|
|
|
17.
|
If a balloon is heated, what happens to the volume of the air in the balloon if
the pressure is constant?
a. | It increases. | c. | It decreases. | b. | It stays the same. | d. | The change cannot be
predicted. |
|
|
|
18.
|
When the volume and number of particles of a gas are constant, which of the
following is also constant?
a. | the sum of the pressure and temperature in kelvins | b. | the difference of
the pressure and temperature in kelvins | c. | the product of the pressure and temperature in
kelvins | d. | the ratio of the pressure and temperature in kelvins |
|
|
|
19.
|
If a sealed syringe is plunged into cold water, in which direction will the
syringe piston slide?
a. | in | c. | No movement will occur. | b. | out | d. | The direction cannot be
predicted. |
|
|
|
20.
|
What happens when a piston is used to decrease the volume of a contained
gas?
a. | Fewer gas particles exert a force on the piston. | b. | The piston’s
pressure on the gas becomes greater than the pressure exerted by the gas on the
piston. | c. | Gas particles become compressed. | d. | Gas particles leak out of the
container. |
|
|
|
21.
|
If a sealed syringe is heated, in which direction will the syringe plunger
move?
a. | out | c. | The plunger will not move. | b. | in | d. | The direction cannot be
predicted. |
|
|
|
22.
|
The combined gas law relates which of the following?
a. | pressure and volume only | c. | volume and temperature
only | b. | temperature and pressure only | d. | temperature, pressure, and
volume |
|
|
|
23.
|
At a certain temperature and pressure, 0.20 mol of carbon dioxide has a volume
of 3.1 L. A 3.1-L sample of hydrogen at the same temperature and pressure ____.
a. | has the same mass | b. | contains the same number of
atoms | c. | has a higher density | d. | contains the same number of
molecules |
|
|
|
24.
|
Which of the following is constant for 1 mole of any ideal gas?
|
|
|
25.
|
At low temperatures and pressures, how does the volume of a real gas compare
with the volume of an ideal gas under the same conditions?
a. | It is greater. | c. | There is no difference. | b. | It is
less. | d. | It depends on the
type of gas. |
|
|
|
26.
|
An ideal gas CANNOT be ____.
a. | condensed | c. | heated | b. | cooled | d. | compressed |
|
|
|
27.
|
Under what conditions of temperature and pressure is the behavior of real gases
most like that of ideal gases?
a. | low temperature and low pressure | c. | high temperature and low
pressure | b. | low temperature and high pressure | d. | high temperature and high
pressure |
|
|
|
28.
|
If the atmospheric pressure on Mt. Everest is one-third the atmospheric pressure
at sea level, the partial pressure of oxygen on Everest is ____.
a. | one-sixth its pressure at sea level | c. | one-half its pressure at sea
level | b. | one-third its pressure at sea level | d. | equal to its pressure at sea
level |
|
|
|
29.
|
What happens to the partial pressure of oxygen in a sample of air if the
temperature is increased?
a. | It increases. | c. | It decreases. | b. | It stays the same. | d. | The change cannot be
determined. |
|
|
|
30.
|
A breathing mixture used by deep-sea divers contains helium, oxygen, and carbon
dioxide. What is the partial pressure of oxygen at 101.4 kPa if 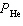 = 82.5 kPa and 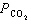 =
0.4 kPa? a. | 82.9 kPa | c. | 18.5 kPa | b. | 19.3 kPa | d. | 101.0 kPa |
|
|
|
31.
|
Which of the following gases will effuse the most rapidly?
a. | bromine | c. | ammonia | b. | chlorine | d. | hydrogen |
|
Short Answer (Value 18)
|
|
|
32.
|
A gas occupies a volume of 140 mL at 35.0 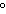 C and 97 kPa. What is the
volume of the gas at STP?
|
|
|
33.
|
A 10-g mass of krypton occupies 15.0 L at a pressure of 210 kPa. Find the volume
of the krypton when the pressure is increased to 790 kPa.
|
|
|
34.
|
A gas storage tank has a volume of 3.5 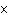 10 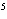 m 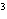 when the
temperature is 27 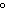 C and the pressure is 101 kPa. What is the new volume of the
tank if the temperature drops to –10 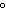 C and the pressure drops to 95 kPa?
|
|
|
35.
|
The gaseous product of a reaction is collected in a 25.0-L container at 27 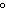 C.
The pressure in the container is 300.0 kPa and the gas has a mass of 96.0 g. How many moles of the
gas are in the container?
|
|
|
36.
|
What is the pressure exerted by 32 g of O 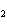 in a 22.0-L
container at 30.0 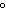 C?
|
|
|
37.
|
A mixture of gases at a total pressure of 95 kPa contains N 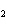 ,
CO 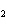 , and O 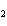 . The partial pressure of the CO 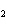 is 24
kPa and the partial pressure of the N 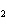 is 48 kPa. What is the partial pressure of the
O 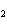 ?
|
Essay (Value 2)
|
|
|
38.
|
How does the pressure of an enclosed gas in a rigid container change when the
gas is heated? Explain why this change occurs.
|