Matching (Value 8)
|
|
|
Match each item with the correct statement below. a. | actual yield | e. | limiting reagent | b. | percent yield | f. | mass | c. | theoretical
yield | g. | number of
molecules | d. | excess reagent | h. | volume |
|
|
|
1.
|
This quantity can always be used in the same way as moles when interpreting
balanced chemical equations.
|
|
|
2.
|
This is conserved only in reactions where the temperature is constant and the
number of moles of gaseous reactants is the same as that of gaseous products. (Answer is h -
volume)
|
|
|
3.
|
This is conserved in every ordinary chemical reaction.
|
|
|
4.
|
the reactant that determines the amount of product that can be formed in a
reaction
|
|
|
5.
|
the maximum amount of product that could be formed from given amounts of
reactants
|
|
|
6.
|
the reactant that is not completely used up in a reaction
|
|
|
7.
|
the amount of product formed when a reaction is carried out in the
laboratory
|
|
|
8.
|
the ratio of the actual yield to the theoretical yield
|
Multiple Choice (Value 18)
Identify the choice that best completes the statement or answers
the question. Show work for all calculation questions.
|
|
|
9.
|
The calculation of quantities in chemical equations is called ____.
a. | stoichiometry | c. | percent composition | b. | dimensional analysis | d. | percent yield |
|
|
|
10.
|
In a chemical reaction, the mass of the products ____.
a. | is less than the mass of the reactants | b. | is greater than the mass of the
reactants | c. | is equal to the mass of the reactants | d. | has no relationship to the mass of the
reactants |
|
|
|
11.
|
In any chemical reaction, the quantities that are preserved are ____.
a. | the number of moles and the volumes | b. | the number of molecules and the
volumes | c. | mass and number of atoms | d. | mass and moles |
|
|
|
12.
|
In the reaction 2CO( g) + O 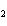 ( g) ® 2CO 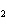 ( g), what is the ratio of moles of
oxygen used to moles of CO 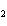 produced?
|
|
|
13.
|
How many moles of glucose, C 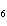 H 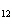 O 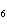 , can be "burned" biologically when
10.0 mol of oxygen is available? C 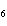 H 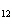 O 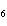 ( s) + 6O 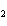 ( g)
® 6CO 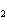 ( g) + 6H 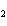 O( l) a. | 0.938 mol | c. | 53.3 mol | b. | 1.67 mol | d. | 60.0 mol |
|
|
|
14.
|
When iron rusts in air, iron(III) oxide is produced. How many moles of oxygen
react with 2.4 mol of iron in the rusting reaction? 4Fe( s) + 3O 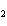 ( g) ® 2Fe2O 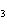 ( s) a. | 1.2 mol | c. | 2.4 mol | b. | 1.8 mol | d. | 3.2 mol |
|
|
|
15.
|
The equation below shows the decomposition of lead nitrate. How many grams of
oxygen are produced when 11.5 g NO 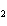 is formed? 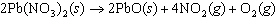 a. | 1.00 g | c. | 2.88 g | b. | 2.00 g | d. | 32.0 g |
|
|
|
16.
|
Iron(III) oxide is formed when iron combines with oxygen in the air. How many
grams of Fe 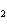 O 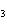 are formed
when 16.7 g of Fe reacts completely with oxygen? 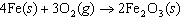 a. | 12.0 g | c. | 47.8 g | b. | 23.9 g | d. | 95.6 g |
|
|
|
17.
|
How many moles of H 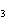 PO 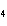
are produced when 71.0 g P 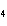 O 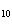 reacts
completely to form H 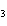 PO 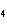 ? 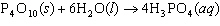 a. | 0.063 5 mol | c. | 4.00 mol | b. | 1.00 mol | d. | 16.0 mol |
|
|
|
18.
|
How many grams of H 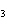 PO 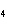
are produced when 10.0 moles of water react with an excess of P 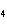 O 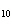 ? 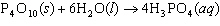 a. | 1.22 g | c. | 147 g | b. | 6.7 g | d. | 653 g |
|
|
|
19.
|
When two substances react to form products, the reactant which is used up is
called the ____.
a. | determining reagent | c. | excess reagent | b. | limiting reagent | d. | catalytic
reagent |
|
|
|
20.
|
How many grams of chromium are needed to react with an excess of CuSO 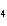 to produce 27.0 g Cu? 2Cr( s) + 3CuSO 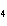 ( aq)
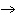 Cr 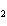 (SO 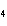 ) 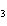 ( aq) + 3Cu( s) a. | 14.7 g | c. | 33.2 g | b. | 18.0 g | d. | 81.5 g |
|
|
|
21.
|
How many grams of beryllium are needed to produce 36.0 g of hydrogen? (Assume an
excess of water.) Be( s) + 2H 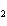 O( l)  Be(OH) 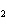 ( aq)
+ H 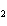 ( g) a. | 4.00 g | c. | 162 g | b. | 36.0 g | d. | 324 g |
|
|
|
22.
|
What is the maximum number of grams of PH 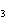 that can be
formed when 6.2 g of phosphorus reacts with 4.0 g of hydrogen to form PH 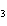 ? P 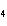 ( g) + 6H 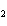 ( g)
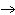 4PH 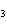 ( g) a. | 0.43 g | c. | 270 g | b. | 6.8 g | d. | 45 g |
|
|
|
23.
|
When an equation is used to calculate the amount of product that will form
during a reaction, then the value obtained is called the ____.
a. | actual yield | c. | theoretical yield | b. | percent yield | d. | minimum yield |
|
|
|
24.
|
Which of the following is NOT a reason why actual yield is less than theoretical
yield?
a. | impure reactants present | c. | loss of product during
purification | b. | competing side reactions | d. | conservation of mass |
|
|
|
25.
|
Lead nitrate can be decomposed by heating. What is the percent yield of the
decomposition reaction if 9.9 g Pb(NO 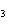 ) 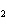 are heated
to give 5.5 g of PbO? 2Pb(NO 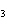 ) 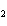 ( s)
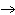 2PbO( s) + 4NO 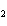 ( g) + O 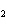 ( g)
|
|
|
26.
|
In a particular reaction between copper metal and silver nitrate, 12.7 g Cu
produced 38.1 g Ag. What is the percent yield of silver in this reaction? Cu + 2AgNO 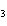  Cu(NO 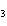 ) 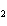 + 2Ag a. | 56.7% | c. | 88.2% | b. | 77.3% | d. | 176% |
|
Short Answer (Value 2)
|
|
|
27.
|
If a tricycle factory ordered 33,432 wheels in 2002 and used all of them,
how many tricycles did the factory produce? (Vehicle)
|