Multiple Choice (Value 10) + 1 Bonus
Identify the choice that best completes the statement or answers
the question.
|
|
|
1.
|
The calculation of quantities in chemical equations is called ____.
a. | stoichiometry | c. | percent composition | b. | dimensional analysis | d. | percent yield |
|
|
|
2.
|
If 1 egg and 1/3 cup of oil are needed for each bag of brownie mix, how many
bags of brownie mix do you need if you want to use up all 3 eggs and 1 cup of oil?
|
|
|
3.
|
What is conserved in the reaction shown below? H 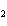 ( g)
+ Cl 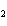 ( g) ® 2HCl( g) a. | mass only | c. | mass, moles, and molecules only | b. | mass and moles
only | d. | mass, moles,
molecules, and volume |
|
|
|
4.
|
What is conserved in the reaction shown below? N 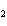 ( g)
+ 3F 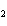 ( g) ® 2NF 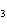 ( g) a. | atoms only | c. | mass and atoms only | b. | mass only | d. | moles only |
|
|
|
5.
|
In every chemical reaction, ____.
a. | mass and molecules are conserved | c. | mass and atoms are
conserved | b. | moles and liters are conserved | d. | moles and molecules are
conserved |
|
|
|
6.
|
In a chemical reaction, the mass of the products ____.
a. | is less than the mass of the reactants | b. | is greater than the mass of the
reactants | c. | is equal to the mass of the reactants | d. | has no relationship to the mass of the
reactants |
|
|
|
7.
|
In any chemical reaction, the quantities that are preserved are ____.
a. | the number of moles and the volumes | b. | the number of molecules and the
volumes | c. | mass and number of atoms | d. | mass and moles |
|
|
|
8.
|
The first step in most stoichiometry problems is to ____.
a. | add the coefficients of the reagents | c. | convert given quantities to
volumes | b. | convert given quantities to moles | d. | convert given quantities to
masses |
|
|
|
9.
|
In the reaction 2CO( g) + O 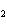 ( g) ® 2CO 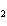 ( g), what is the ratio of moles of oxygen used
to moles of CO 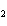 produced?
|
|
|
10.
|
Which of the following is true about the total number of reactants and the total
number of products in the reaction shown below? C 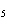 H 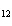 ( l) +
8O 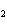 ( g) ® 5CO 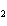 ( g) +
6H 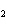 O( g) a. | 9 moles of reactants chemically change into 11 moles of product. | b. | 9 grams of reactants
chemically change into 11 grams of product. | c. | 9 liters of reactants chemically change into 11
liters of product. | d. | 9 atoms of reactants chemically change into 11
atoms of product. |
|
|
|
11.
|
Which of the following is an INCORRECT interpretation of the balanced equation
shown below? 2S( s) + 3O 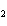 ( g) ®
2SO 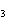 ( g) a. | 2 atoms S + 3 molecules O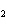 ® 2
molecules SO ® 2
molecules SO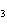 | b. | 2 g S + 3 g O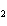 ® 2 g SO ® 2 g SO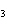 | c. | 2 mol S + 3 mol O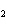 ® 2 mol SO ® 2 mol SO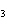 | d. | none of the
above |
|
Numeric Response (Value 9)
|
|
|
12.
|
LiOH + HBr à LiBr +
H2O
If you
start with ten grams of lithium hydroxide, how many grams of lithium bromide will be
produced?
|
|
|
13.
|
C2H4
+ 3 O2 à 2 CO2 + 2
H2O
If you start with 45 grams of
ethylene (C2H4), how many grams of carbon dioxide will be
produced?
|
|
|
14.
|
2 HCl +
Na2SO4 à 2 NaCl +
H2SO4
If you start with 20 grams of hydrochloric acid, how many grams of sulfuric
acid will be produced?
|