Multiple Choice (Value 18)
Identify the choice that best completes the statement or answers
the question.
|
|
|
1.
|
Which of the following statements is NOT correct?
a. | The only way to determine the products of a reaction is to carry out the
reaction. | b. | A single reactant is the identifying characteristic of a decomposition
reaction. | c. | Complete combustion has occurred when all the carbon in the product is in the form of
carbon dioxide. | d. | All chemical reactions can be classified as one of five general
types. |
|
|
|
2.
|
Which of the following statements is true about single-replacement
reactions?
a. | Any metal replaces any other metal. | c. | Two reactants produce two
products. | b. | They involve a single product. | d. | They are restricted to
metals. |
|
|
|
3.
|
Chemical equations ____.
a. | show how to write chemical formulas | b. | describe chemical reactions | c. | give directions for
naming chemical compounds | d. | describe only biological
changes |
|
|
|
4.
|
Chemical reactions ____.
a. | only occur outside living organisms | c. | create and destroy
atoms | b. | produce new substances | d. | occur only in living organisms |
|
|
|
5.
|
The products of a combustion reaction do NOT include ____.
a. | hydrogen | c. | water | b. | carbon dioxide | d. | carbon monoxide |
|
|
|
6.
|
A catalyst is ____.
a. | the product of a combustion reaction | b. | a solid product of a
reaction | c. | one of the reactants in single-replacement reactions | d. | not used up in a
reaction |
|
|
|
7.
|
The product of a combination reaction is Ba(OH) 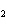 . If
one of the reactants is H 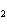 O, what is the other reactant? a. | BaO | c. | BaH | b. | BaO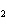 | d. | Ba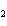 O O |
|
|
|
8.
|
The equation 2C 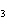 H 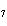 OH 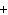 9O 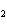 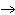 6CO 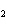 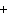 8H 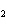 O is an example of which type of reaction? a. | single-replacement reaction | c. | combustion
reaction | b. | double-replacement reaction | d. | decomposition reaction |
|
|
|
9.
|
A double-replacement reaction takes place when aqueous cobalt(III) chloride
reacts with aqueous lithium hydroxide. One of the products of this reaction is ____.
a. | Co(OH)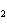 | c. | LiCl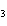 | b. | LiCo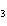 | d. | Co(OH)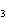 |
|
|
|
10.
|
If you rewrite the following word equation as a balanced chemical equation, what
will the coefficient and symbol for fluorine be? nitrogen trifluoride 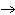 nitrogen 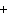 fluorine
|
|
|
11.
|
What are the correct formulas and coefficients for the products of the following
double-replacement reaction? RbOH 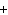 H 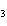 PO 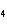 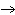
|
|
|
12.
|
The reaction 2Fe 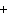 3Cl 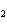 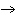 2FeCl 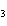 is an
example of which type of reaction? a. | combination reaction | c. | decomposition reaction | b. | combustion
reaction | d. | single-replacement
reaction |
|
|
|
13.
|
In a double-replacement reaction, ____.
a. | one of the reactants is often water | b. | the reactants are usually a metal and a
nonmetal | c. | energy in the form of heat or light is often produced | d. | the reactants are
generally two ionic compounds in aqueous solution |
|
|
|
14.
|
Symbols used in equations, together with the explanations of the symbols, are
shown below. Which set is correct?
a. | (g), grams | c. | (l), liters | b. | (aq), dissolved in
water | d. | (s), solid
product |
|
|
|
15.
|
Which of the following statements is NOT true about the decomposition of a
simple binary compound?
a. | The reactant could be an ionic or a molecular compound. | b. | The reactant is a
single substance. | c. | The products are the constituent
elements. | d. | The products are unpredictable. |
|
|
|
16.
|
Which of the following statements is NOT true about what happens in all chemical
reactions?
a. | New atoms are formed as products. | b. | The bonds of the reactants are broken and new
bonds of the products are formed. | c. | The starting substances are called
reactants. | d. | The ways in which atoms are joined together are
changed. |
|
|
|
17.
|
Which of the following statements is NOT true about double-replacement
reactions?
a. | The product may be a molecular compound. | b. | The product may
precipitate from solution. | c. | The product may be a gas. | d. | The reactant may be
a solid metal. |
|
|
|
18.
|
What does the symbol  in a
chemical equation mean? a. | Heat is supplied to the reaction. | c. | A catalyst is
needed. | b. | yields | d. | precipitate |
|