Multiple Choice (Value 11)
Identify the choice that best completes the statement or answers
the question.
|
|
|
1.
|
When iron oxide becomes iron, what type of reaction occurs?
a. | oxidation | c. | neutralization | b. | reduction | d. | combination |
|
|
|
2.
|
Oxidation is ____.
a. | a loss of oxygen | c. | a loss of electrons | b. | a gain of electrons | d. | a gain of
hydrogen |
|
|
|
3.
|
What are transferred in an oxidation-reduction reaction?
a. | protons | c. | electrons | b. | ions | d. | atoms |
|
|
|
4.
|
In the reaction of calcium with chlorine, which atom is the oxidizing
agent?
a. | calcium | c. | both a and b | b. | chlorine | d. | neither a nor b |
|
|
|
5.
|
In the reaction of calcium with chlorine, which atom is oxidized?
a. | calcium | c. | both a and b | b. | chlorine | d. | neither a nor b |
|
|
|
6.
|
In the reaction of hydrogen with iodine, which atom is oxidized?
a. | hydrogen | c. | both a and b | b. | iodine | d. | neither a nor b |
|
|
|
7.
|
In the reaction of hydrogen with iodine, which atom is the reducing
agent?
a. | hydrogen | c. | both a and b | b. | iodine | d. | neither a nor b |
|
|
|
8.
|
What is the reducing agent in the following reaction? 2Na + S ® Na 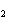 S a. | Na | c. | Na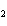 S S | b. | S | d. | Na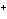 |
|
|
|
9.
|
Which statement is true about the following reaction? S + Cl 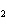 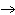 SCl 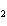 (Hint: Chlorine is the more
electronegative element.) a. | Sulfur is reduced to SCl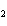 . . | c. | Chlorine is oxidized to SCl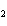 . . | b. | Chlorine is reduced to SCl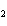 . . | d. | Sulfur is the oxidizing
agent. |
|
|
|
10.
|
What is the oxidizing agent in the following reaction? I 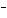 + MnO 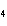 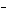 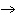 I 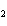 + MnO 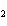
|
|
|
11.
|
Why is oxygen reduced in the reaction of hydrogen with oxygen to make
water?
a. | Oxygen pulls electrons toward itself. | b. | Oxygen pushes electrons toward the
hydrogens. | c. | Oxygen absorbs a proton. | d. | Oxygen releases a
proton. |
|