Multiple Choice (Value 13)
Identify the choice that best completes the statement or answers
the question.
|
|
|
1.
|
At equilibrium, what is the rate of production of reactants compared with the
rate of production of products?
a. | much higher | c. | the same | b. | higher | d. | lower |
|
|
|
2.
|
If a reaction is reversible, what are the relative amounts of reactant and
product at the end of the reaction?
a. | no reactant; all product | b. | no product; all reactant | c. | some product; some
reactant | d. | The relationship between reactants and products cannot be
determined. |
|
|
|
3.
|
If sulfur dioxide and oxygen can be made into sulfur trioxide, what is the
reverse reaction?
|
|
|
4.
|
Consider the reaction N 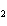 ( g) 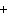 3H 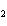 ( g)
 2NH 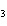 ( g). What is the effect of decreasing the volume
on the contained gases? a. | The reaction shifts toward the product gas. | b. | The system reacts by
increasing the number of gas molecules. | c. | The pressure on the gases decreases
momentarily. | d. | Ammonia is consumed in the reaction. |
|
|
|
5.
|
What happens to a reaction at equilibrium when more reactant is added to the
system?
a. | The reaction makes more products. | c. | The reaction is
unchanged. | b. | The reaction makes more reactants. | d. | The answer cannot be
determined. |
|
|
|
6.
|
In an endothermic reaction at equilibrium, what is the effect of raising the
temperature?
a. | The reaction makes more products. | c. | The reaction is
unchanged. | b. | The reaction makes more reactants. | d. | The answer cannot be
determined. |
|
|
|
7.
|
In a reaction (at equilibrium) that makes more moles of gas than it consumes,
what is the effect of increasing the pressure?
a. | The reaction makes more products. | c. | The reaction is
unchanged. | b. | The reaction makes more reactants. | d. | The answer cannot be
determined. |
|
|
|
8.
|
Which of the changes listed below would shift the following reaction to the
right? 4HCl( g) + O 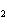 ( g)  2Cl 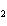 ( g) + 2H 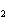 O( g) a. | addition of Cl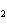 | c. | increase of
pressure | b. | removal of O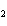 | d. | decrease of
pressure |
|
|
|
9.
|
What is the effect of adding more water to the following equilibrium
reaction? CO 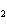 + H 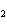 O  H 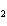 CO 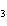 a. | More H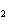 CO CO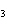 is produced. is produced. | b. | CO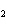 concentration increases.
concentration increases. | c. | The equilibrium is pushed in the direction of
reactants. | d. | There is no effect. |
|
|
|
10.
|
What is the equilibrium constant for the following reaction? C + O 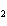
 CO 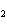
|
|
|
11.
|
If a reaction has an equilibrium constant just greater than 1, what type of
reaction is it?
a. | irreversible | c. | reversible, favoring products | b. | spontaneous | d. | reversible, favoring reactants |
|
|
|
12.
|
In an equilibrium reaction with a K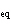 of 1 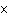 10 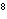 ,
the ____. a. | reactants are favored | c. | the products are favored | b. | reaction is
spontaneous | d. | reaction is
exothermic |
|
|
|
13.
|
The K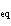 of a reaction is 4 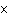 10 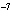 . At
equilibrium, the ____. a. | reactants are favored | b. | products are favored | c. | reactants and
products are present in equal amounts | d. | rate of the forward reaction is much greater
than the rate of the reverse reaction |
|
Short Answer (Value 8)
|
|
|
14.
|
What is the equilibrium constant for the following reaction? Si + O 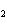
 SiO 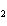
|
|
|
15.
|
What is the equilibrium constant for the following reaction? 3A + 2B 
2C
|
|
|
16.
|
A mixture of hydrogen and iodine are in equilibrium with hydrogen iodide, as
shown in the following equation. H 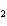 + I 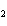  2HI Calculate the
concentration of HI when the equilibrium constant is 1 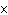 10 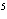 , the equilibrium
concentration of H 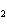 is 0.04 M, and the equilibrium concentration of
I 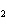 is 0.009 M.
|
|
|
17.
|
Calculate the value of K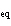 for the following reaction at
equilibrium. 2NClO( g)  2NO( g) + Cl 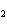 ( g) An
analysis of the equilibrium mixture in a 1-L flask gives the following results: NClO, 1.6 mol; NO,
6.4 mol; Cl 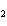 , 0.49 mol
|