Matching (Value 25)
|
|
|
Match each item with the correct statement below. a. | functional group | f. | halogen | b. | hydroxyl group | g. | fatty acids | c. | carbonyl
group | h. | alcohol | d. | carboxyl group | i. | glycerol | e. | ether
|
|
|
|
1.
|
a carbonyl group attached to a hydroxyl group
|
|
|
2.
|
a specific arrangement of atoms in an organic compound that is capable of
characteristic chemical reactions
|
|
|
3.
|
a compound in which oxygen is bonded to two carbon atoms
|
|
|
4.
|
a carbon atom and an oxygen atom joined by a double bond
|
|
|
5.
|
reacts with a carboxylic acid to form an ester
|
|
|
6.
|
the OH functional group in alcohols
|
|
|
7.
|
carboxylic acids with long hydrocarbon chains
|
|
|
8.
|
reacts with an alkane by a substitution reaction
|
|
|
9.
|
a main component of fats and oils
|
|
|
Match each item with the correct statement below. a. | aromatic compound | d. | lignite | b. | aliphatic hydrocarbon | e. | bituminous coal | c. | anthracite
coal |
|
|
|
10.
|
soft coal, having a carbon content of 70–80%
|
|
|
11.
|
hard coal, having a carbon content of over 80%
|
|
|
12.
|
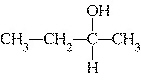
brown coal, having a carbon content of approximately 50%
|
|
|
13.
|
any straight-chain or branched-chain alkane, alkene, or alkyne
|
|
|
Match each item with the correct statement below. a. | substituent | e. | asymmetric carbon | b. | structural isomers | f. | trans configuration | c. | geometric
isomers | g. | cis
configuration | d. | stereoisomers |
|
|
|
14.
|
arrangement in which substituted groups are on the same side of a double
bond
|
|
|
15.
|
arrangement in which substituted groups are on opposite sides of a double
bond
|
|
|
16.
|
atom or group of atoms that can take the place of a hydrogen in a parent
hydrocarbon molecule
|
|
|
17.
|
compounds that differ in the orientation of groups around a double bond
|
|
|
18.
|
carbon atom to which four different atoms or groups are attached
|
|
|
19.
|
compounds that have the same molecular formula, but the atoms are joined in a
different order
|
|
|
20.
|
molecules in which atoms are joined in the same order but differ in the
arrangements of their atoms in space
|
|
|
Match each item with the correct statement below. a. | substitution reaction | d. | hydrogenation reaction | b. | addition
reaction | e. | dehydrogenation
reaction | c. | hydration reaction |
|
|
|
21.
|
a reaction involving the loss of hydrogen
|
|
|
22.
|
a reaction in which an atom or group of atoms replaces another atom or group of
atoms
|
|
|
23.
|
a reaction in which a substance is added at the double or triple bond of an
alkene or alkyne
|
|
|
24.
|
a reaction involving the addition of hydrogen to a carbon—carbon double
bond to produce an alkane
|
|
|
25.
|
a reaction involving the addition of water to an alkene
|
Multiple Choice (Value 75)
Identify the choice that best completes the statement or answers
the question.
|
|
|
26.
|
The specific heat of silver is 0.24 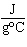 . How many joules of energy are
needed to warm 4.37 g of silver from 25.0 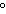 C to 27.5 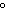 C? a. | 45.5 J | c. | 2.62 J | b. | 0.14 J | d. | 0.022 J |
|
|
|
27.
|
Which of the following compounds is the most soluble in water?
a. | l-bromopropane | c. | propanoic acid | b. | propane | d. | propanal |
|
|
|
28.
|
How does a calorie compare to a joule?
a. | A calorie is smaller than a joule. | c. | A calorie is equal to a
joule. | b. | A calorie is larger than a joule. | d. | The relationship cannot be
determined. |
|
|
|
29.
|
Which of the following substances has the highest specific heat?
a. | steel | c. | alcohol | b. | chloroform | d. | water |
|
|
|
30.
|
What is the heat of solution?
a. | the amount of heat required to change a solid into a liquid | b. | the amount of heat
required to change a vapor into a liquid | c. | the amount of heat absorbed or released when a
solid dissolves | d. | the amount of heat released when a vapor changes into a
liquid |
|
|
|
31.
|
Phenols are characterized by ____.
a. | their use as flavoring agents | c. | an ¾OH group on a benzene ring | b. | their behavior as gases | d. | ether linkages |
|
|
|
32.
|
The most important way to classify organic compounds is by ____.
a. | functional group | b. | the type of carbon—carbon
bonds | c. | reactivity | d. | the number of carbon atoms in the longest
chain |
|
|
|
33.
|
What is the simplest alkane?
a. | pentane | c. | butane | b. | methane | d. | ethane |
|
|
|
34.
|
The longest continuous carbon chain of a branched-chain hydrocarbon is called
a(n) ____.
a. | substituted alkane | c. | parent alkane | b. | principle alkane | d. | isomer |
|
|
|
35.
|
 H H for the formation of rust
(Fe 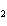 O 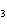 ) is –826 kJ/mol. How much
energy is involved in the formation of 5 grams of rust? a. | 66 J | c. | 25.9 J | b. | 66 kJ | d. | 25.9 kJ |
|
|
|
36.
|
Standard conditions of temperature and pressure for a thermochemical equation
are ____.
a. | 25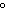 C and 101 kPa C and 101 kPa | c. | 0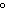 C and 101
kPa C and 101
kPa | b. | 25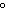 C and 22.4 kPa C and 22.4 kPa | d. | 0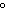 C and 0
kPa C and 0
kPa |
|
|
|
37.
|
What is the amount of heat required to raise the temperature of 200.0 g of
aluminum by 10 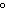 C? (specific heat of aluminum = 0.21 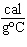 ) a. | 420,000 cal | c. | 42,000 cal | b. | 420 cal | d. | 4200 cal |
|
|
|
38.
|
What happens to a catalyst in a reaction?
a. | It is incorporated into the reactants. | c. | It is
unchanged. | b. | It evaporates away. | d. | It is incorporated into the products. |
|
|
|
39.
|
The quantity of heat required to change the temperature of 1 g of a substance by
1 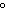 C is defined as ____. a. | a joule | c. | specific heat | b. | density | d. | a calorie |
|
|
|
40.
|
Which carbon skeleton represents a ketone?
|
|
|
41.
|
What would likely happen if you were to touch the flask in which an endothermic
reaction were occurring?
a. | The flask would probably feel warmer than before the reaction
started. | b. | The flask would probably feel cooler than before the reaction
started. | c. | The flask would feel the same as before the reaction started. | d. | none of the
above |
|
|
|
42.
|
What substance is added to an organic molecule to test for the degree of
saturation?
a. | hydrogen gas | c. | bromine | b. | water | d. | hydrogen
bromide |
|
|
|
43.
|
What is the increment of change in a series of straight-chain alkanes?
|
|
|
44.
|
Which of the following is NOT a fraction obtained from crude oil?
a. | natural gas | c. | gasoline | b. | ammonia | d. | kerosene |
|
|
|
45.
|
A saturated straight-chain hydrocarbon with two carbons is ____.
a. | ethane | c. | decane | b. | propane | d. | ethene |
|
|
|
46.
|
The  H H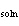 is ____. a. | always positive | b. | always negative | c. | sometimes positive,
sometimes negative | d. | always 0 |
|
|
|
47.
|
Which carbon skeleton represents an ester?
|
|
|
48.
|
What happens to the energy produced by burning gasoline in a car engine?
a. | The energy is lost as heat in the exhaust. | b. | The energy is
transformed into work to move the car. | c. | The energy heats the parts of the
engine. | d. | all of the above |
|
|
|
49.
|
Which of the following is a condensed structural formula for propane?
|
|
|
50.
|
Which of the following is NOT a product obtained from the distillation of coal
tar?
a. | coke | c. | toluene | b. | phenol | d. | benzene |
|
|
|
51.
|
The amount of heat involved in the synthesis of 1 mole of a compound from its
elements, with all substances in their standard states at 25 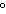 C, is called ____. a. | heat of solidification | c. | enthalpy | b. | standard heat of formation | d. | heat of
reaction |
|
|
|
52.
|
When 45 g of an alloy, at 25 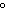 C, are dropped into 100.0 g of
water, the alloy absorbs 956 J of heat. If the final temperature of the alloy is 37 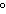 C,
what is its specific heat? a. | 1.77 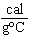 | c. | 0.423 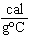 | b. | 48.8 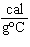 | d. | 9.88 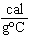 |
|
|
|
53.
|
How many double covalent bonds are in an alkane?
|
|
|
54.
|
How can you describe the specific heat of olive oil if it takes approximately
420 J of heat to raise the temperature of 7 g of olive oil by 30 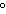 C? a. | less than the specific heat of water | c. | Not enough information is
given. | b. | equal to the specific heat of water | d. | greater than the specific heat of
water |
|
|
|
55.
|
Which of the following carbon skeletons represents a carboxylic acid?
|
|
|
56.
|
Which of the following compounds will produce the least energy when completely
oxidized?
a. | hexanal | c. | hexanol | b. | hexanoic acid | d. | hexane |
|
|
|
57.
|
What happens in a condensation reaction?
a. | cross-linking of monomers | b. | head-to-tail joining of
monomers | c. | side-by-side joining of monomers | d. | substitution of a halogen on
monomers |
|
|
|
58.
|
Which carbon skeleton contains a carboxyl group?
|
|
|
59.
|
What is the carbon skeleton of the product formed in the following reaction?
C 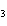 H 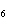 + HBr ® a. |
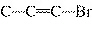 | c. |
C¾C¾C¾Br | b. |
C¾C¾Br¾C | d. |
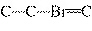 |
|
|
|
60.
|
When 10 g of diethyl ether is converted to vapor at its boiling point, about how
much heat is absorbed? (C 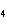 H 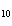 O,  H H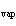 = 15.7 kJ/mol, boiling point: 34.6 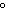 C) a. | 2 J | c. | Not enough information is given. | b. | 0.2 kJ | d. | 2 kJ |
|
|
|
61.
|
The functional group in CH 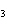 ¾ ¾O ¾CH 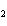 ¾ ¾CH 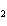 ¾ ¾CH 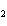 ¾ ¾CH 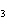 is a(n) ____. a. | ester | c. | ether | b. | carbonyl | d. | carboxyl |
|
|
|
62.
|
What is the common name of the following alcohol? 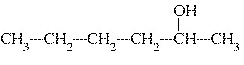 a. | tert-hexyl alcohol | c. | sec-hexyl
alcohol | b. | isohexyl alcohol | d. | hexyl alcohol |
|
|
|
63.
|
By what quantity must the heat capacity of an object be divided to obtain the
specific heat of that material?
a. | its volume | c. | its energy | b. | its mass | d. | its temperature |
|
|
|
64.
|
Hydrocarbons containing a saturated carbon ring are called ____.
a. | alkylated hydrocarbons | c. | aromatic hydrocarbons | b. | aliphatic
hydrocarbons | d. | cyclic
hydrocarbons |
|
|
|
65.
|
The names of the straight-chain alkanes all end with the suffix ____.
|
|
|
66.
|
How many kilocalories of heat are required to raise the temperature of 225 g of
aluminum from 20 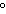 C to 100 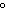 C? (specific heat of aluminum =
0.21 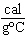 ) a. | 3.8 kcal | c. | 0.59 kcal | b. | 85 kcal | d. | none of the
above |
|
|
|
67.
|
Which of the following compounds is an unsaturated hydrocarbon?
a. | methane | c. | propyne | b. | nonane | d. | methyl |
|
|
|
68.
|
How many carbons are in a molecule of hexane?
|
|
|
69.
|
Why does a higher concentration make a reaction faster?
a. | There are more collisions per second or the collisions are of greater
energy. | b. | There are more collisions per second only. | c. | Collisions occur
with greater energy only. | d. | There are more collisions per second and the
collisions are of greater energy. |
|
|
|
70.
|
The heat capacity of an object depends in part on its ____.
a. | potential energy | c. | shape | b. | enthalpy | d. | mass |
|
|
|
71.
|
An organic compound that contains only carbon and hydrogen and at least one
carbon-carbon triple bond is classified as an ____.
a. | alkene | c. | arene | b. | alkane | d. | alkyne |
|
|
|
72.
|
The general name for hydrocarbons with at least one triple covalent bond is
____.
a. | alkyls | c. | alkynes | b. | alkanes | d. | alkenes |
|
|
|
73.
|
What is the general formula for a straight-chain alkane?
|
|
|
74.
|
The name for an alkyl group that contains two carbon atoms is ____.
a. | ethyl | c. | dimethyl | b. | propyl | d. | diphenyl |
|
|
|
75.
|
The amount of heat needed to melt one mole of a solid at a constant temperature
is called ____.
a. | enthalpy | c. | molar heat of solidification | b. | heat of
reaction | d. | molar heat of
fusion |
|
|
|
76.
|
The symbol  H H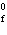 stands for the ____. a. | heat capacity of a substance | b. | specific heat of a
substance | c. | heat of reaction for a chemical reaction | d. | standard heat of
formation for a compound |
|
|
|
77.
|
A structural isomer of hexane is ____.
a. | cyclohexane | c. | benzene | b. | 2,2-dimethylbutane | d. | 2-methylpentene |
|
|
|
78.
|
Which of the following has the greatest heat capacity?
a. | 1 g of water | c. | 1 g of steel | b. | 1000 g of water | d. | 1000 g of steel |
|
|
|
79.
|
Esters contribute which property to fruits?
a. | odor | c. | skin thickness | b. | color | d. | texture |
|
|
|
80.
|
A piece of metal is heated, then submerged in cool water. Which statement below
describes what happens?
a. | The temperature of the water will decrease. | b. | The temperature of
the water will increase. | c. | The temperature of the water will increase and
the temperature of the metal will decrease. | d. | The temperature of the metal will
increase. |
|
|
|
81.
|
What type of compound is the following? 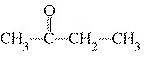 a. | aldehyde | c. | alcohol | b. | ether | d. | ketone |
|
|
|
82.
|
Which hydrocarbon rings are most common in nature?
a. | rings with 3 or 4 carbon atoms | c. | rings with 4 or 5 carbon
atoms | b. | rings with 5 or 6 carbon atoms | d. | rings with 6 or 7 carbon
atoms |
|
|
|
83.
|
Calculate the energy required to produce 7.00 mol Cl 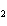 O 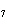 on
the basis of the following balanced equation. 2Cl 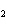 ( g) + 7O 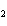 ( g) + 130 kcal 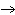 2Cl 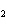 O 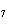 ( g) a. | 130 kcal | c. | 7.00 kcal | b. | 455 kcal | d. | 65 kcal |
|
|
|
84.
|
Which of the following compounds is a secondary alcohol?
a. |
CH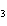 ¾CH ¾CH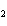 ¾CH ¾CH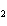 ¾CH ¾CH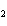 OH OH | c. |
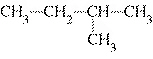 | b. |
 | d. |
none of the
above |
|
|
|
85.
|
Which of the following is a valid unit for specific heat?
|
|
|
86.
|
Alkanes are hydrocarbons that contain what type of bonds?
a. | ionic bonds | c. | single covalent bonds only | b. | at least one double
bond | d. | at least one triple
bond |
|
|
|
87.
|
The IUPAC name for a carboxylic acid with two carbons in a straight chain would
be ____.
a. | methacarboxylic acid | c. | ethanalic acid | b. | ethanoic acid | d. | dimethylmethanoic
acid |
|
|
|
88.
|
Which of the following substances act as catalysts in the body?
a. | carbohydrates | c. | enzymes | b. | nucleic acids | d. | lipids |
|
|
|
89.
|
Why are the molecules of hydrocarbons nonpolar?
a. | The intermolecular attractions are strong. | b. | The electron pair is
shared almost equally in all the bonds. | c. | Van der Waals forces overcome
polarity. | d. | All the bonds are single covalent bonds. |
|
|
|
90.
|
Which of the following compounds has the highest boiling point?
a. | butane | c. | butanal | b. | ethyl acetate | d. | butanoic acid |
|
|
|
91.
|
What type of compound is CH 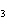 ¾ ¾O ¾CH 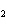 ¾ ¾CH 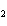 ¾ ¾CH 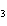 ? a. | aldehyde | c. | ketone | b. | alcohol | d. | ether |
|
|
|
92.
|
What is the standard heat of reaction for the following
reaction? Zn( s) + Cu 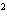 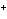 ( aq) 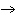 Zn 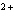 ( aq) +
Cu( s) (  H H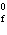 for Cu 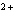 =
+64.4 kJ/mol;  H H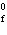 for Zn 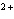 =
–152.4 kJ/mol) a. | 88.0 kJ absorbed per mole | c. | 216.8 kJ released per
mole | b. | 88.0 kJ released per mole | d. | 216.8 kJ absorbed per mole |
|
|
|
93.
|
How are hydrogen atoms arranged in ethene?
a. | in different planes, separated by angles of 120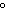 | b. | in the same plane, separated by angles of 180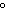 | c. | in different planes, separated by angles of 180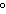 | d. | in the same plane, separated by angles of 120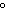 |
|
|
|
94.
|
Which of the following is NOT an important fossil fuel?
a. | hydrogen | c. | petroleum | b. | coal | d. | natural gas |
|
|
|
95.
|
Why does a catalyst cause a reaction to proceed faster?
a. | The activation energy is lowered only. | b. | There are more collisions per second
only. | c. | The collisions occur with greater energy only. | d. | There are more
collisions per second and the collisions are of greater energy. |
|
|
|
96.
|
Calculate the energy released when 24.8 g Na 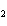 O reacts in the
following reaction. Na 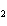 O( s) + 2HI( g) 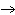 2NaI( s) + H 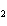 O( l)  H H = –120.00 kcal a. | 2.42 kcal | c. | 3.00 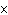 10 10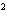 kcal kcal | b. | 48.0
kcal | d. | 0.207
kcal |
|
|
|
97.
|
When 1.0 g of solid NaOH (  H H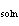 =
–445.1 kJ/mol) dissolves in 10 L of water, how much heat is released? a. | 445.1 kJ | c. | 405.1 kJ | b. | 11.1 J | d. | 11.1 kJ |
|
|
|
98.
|
How many covalent bonds can each carbon atom form?
|
|
|
99.
|
If heat is released by a chemical system, an equal amount of heat will be
____.
a. | released by the universe | c. | absorbed by the
surroundings | b. | released by the surroundings | d. | absorbed by the
universe |
|
|
|
100.
|
A piece of candy has 5 Calories (or 5000 calories). If it could be burned,
leaving nothing but carbon dioxide and water, how much heat would it give off?
a. | 5 kilocalories | c. | Not enough information is given. | b. | 500
calories | d. | 5000
joules |
|
Short Answer (Value 9)
|
|
|
101.
|
How many joules are there in 215 calories? (1 cal = 4.184 J)
|
|
|
102.
|
Write the general structure for halocarbon compounds.
|
|
|
103.
|
It takes 770 joules of energy to raise the temperature of 50.0 g of mercury by
110  C. What is the specific heat of mercury?
|