Matching (Value 10)
|
|
|
Match each item with the correct statement below. a. | substituent | e. | asymmetric carbon | b. | structural isomers | f. | trans configuration | c. | geometric
isomers | g. | cis
configuration | d. | stereoisomers |
|
|
|
1.
|
atom or group of atoms that can take the place of a hydrogen in a parent
hydrocarbon molecule
|
|
|
2.
|
compounds that have the same molecular formula, but the atoms are joined in a
different order
|
|
|
3.
|
arrangement in which substituted groups are on the same side of a double
bond
|
|
|
4.
|
molecules in which atoms are joined in the same order but differ in the
arrangements of their atoms in space
|
|
|
5.
|
compounds that differ in the orientation of groups around a double bond
|
|
|
6.
|
carbon atom to which four different atoms or groups are attached
|
|
|
Match each item with the correct statement below. a. | aromatic compound | d. | lignite | b. | aliphatic hydrocarbon | e. | bituminous coal | c. | anthracite
coal |
|
|
|
7.
|
any straight-chain or branched-chain alkane, alkene, or alkyne
|
|
|
8.
|
hard coal, having a carbon content of over 80%
|
|
|
9.
|
brown coal, having a carbon content of approximately 50%
|
|
|
10.
|
soft coal, having a carbon content of 70–80%
|
Multiple Choice (Value 22)
Identify the choice that best completes the statement or answers
the question.
|
|
|
11.
|
How many covalent bonds can each carbon atom form?
|
|
|
12.
|
Which of the following is a condensed structural formula for propane?
|
|
|
13.
|
What is the physical state of the smallest alkanes at room temperature?
a. | gas | c. | solid | b. | liquid | d. | gas or liquid |
|
|
|
14.
|
What is the increment of change in a series of straight-chain alkanes?
|
|
|
15.
|
The condensed structural formula for 2,2,3-trimethylbutane is ____.
|
|
|
16.
|
In which of the following liquids is hexane most likely to dissolve?
a. | aqueous ammonium hydroxide | c. | rubbing alcohol | b. | vinegar | d. | octane |
|
|
|
17.
|
An organic compound that contains only carbon and hydrogen and at least one
carbon-carbon triple bond is classified as an ____.
a. | alkane | c. | alkyne | b. | alkene | d. | arene |
|
|
|
18.
|
How are hydrogen atoms arranged in ethene?
a. | in the same plane, separated by angles of 120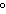 | b. | in different planes, separated by angles of 120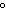 | c. | in the same plane, separated by angles of 180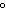 | d. | in different planes, separated by angles of 180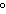 |
|
|
|
19.
|
Which of the following compounds is a structural isomer of butane?
a. | 2-methylbutane | c. | 2-methylpropane | b. | 2,2-dimethylbutane | d. | 2,2-diethylpropane |
|
|
|
20.
|
A structural isomer of hexane is ____.
a. | 2,2-dimethylbutane | c. | benzene | b. | cyclohexane | d. | 2-methylpentene |
|
|
|
21.
|
Alkanes do not have geometric isomers because the carbon atoms in their
carbon-carbon bonds are ____.
a. | double bonds | c. | free to rotate | b. | quite polar | d. | asymmetric |
|
|
|
22.
|
Which hydrocarbon rings are most common in nature?
a. | rings with 3 or 4 carbon atoms | c. | rings with 5 or 6 carbon
atoms | b. | rings with 4 or 5 carbon atoms | d. | rings with 6 or 7 carbon
atoms |
|
|
|
23.
|
Which of the following molecules does NOT display resonance?
a. | benzene | c. | m-xylene | b. | phenylethane | d. | cyclohexane |
|
|
|
24.
|
Which of the following is NOT a fraction obtained from crude oil?
a. | ammonia | c. | gasoline | b. | natural gas | d. | kerosene |
|
|
|
25.
|
Which of the following is NOT a product obtained from the distillation of coal
tar?
a. | benzene | c. | coke | b. | phenol | d. | toluene |
|
|
|
26.
|
Which halocarbon has the highest boiling point?
a. | 1-chloropropane | c. | 1,2,3-trichloropropane | b. | 2-chloropropane | d. | 2-dichloropropane |
|
|
|
27.
|
Which of the following compounds is trichloromethane?
|
|
|
28.
|
Which of the following compounds is a glycol?
|
|
|
29.
|
Which pair of formulas represents the same compound?
|
|
|
30.
|
In an addition reaction, which bond of the reactant is broken?
a. | carbon—carbon single bond | c. | carbon—carbon double
bond | b. | carbon—hydrogen single bond | d. | carbon—hydrogen double
bond |
|
|
|
31.
|
Which of these compounds would you expect to be most soluble in water?
|
|
|
32.
|
Which carbon skeleton represents an ether?
a. |
C¾C¾C¾O¾C¾C¾C | c. |
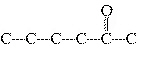 | b. |
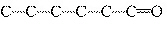 | d. |
none of the
above |
|
Short Answer (Value 3)
|
|
|
33.
|
How many more hydrogen atoms does a cyclohexane molecule have than a benzene
molecule?
|
|
|
34.
|
Write the general structure for ester compounds.
|
|
|
35.
|
Complete the condensation polymerization reaction between two amino acids to
form a peptide bond: 
|
Numeric Response (Value 3)
|
|
|
36.
|
How many arrangements are possible for two methyl groups with respect to a rigid
double bond?
|
|
|
37.
|
How many forms of coal are there?
|
|
|
38.
|
What percent of the composition of natural gas is methane?
|
Essay (Value 4)
|
|
|
39.
|
Describe in your own words what the difference is between unsaturated and
saturated hydrocarbons. What is a saturated compound saturated with?
|
|
|
40.
|
Why is benzene not as reactive as other six-carbon alkenes?
|