Matching (Value 10)
|
|
|
Match each item with the correct statement below. a. | functional group | f. | halogen | b. | hydroxyl group | g. | fatty acids | c. | carbonyl
group | h. | alcohol | d. | carboxyl group | i. | glycerol | e. | ether
|
|
|
|
1.
|
a main component of fats and oils
|
|
|
2.
|
a carbon atom and an oxygen atom joined by a double bond
|
|
|
3.
|
a carbonyl group attached to a hydroxyl group
|
|
|
4.
|
a compound in which oxygen is bonded to two carbon atoms
|
|
|
5.
|
carboxylic acids with long hydrocarbon chains
|
|
|
6.
|
reacts with a carboxylic acid to form an ester
|
|
|
Match each item with the correct statement below. a. | substitution reaction | d. | hydrogenation reaction | b. | addition
reaction | e. | dehydrogenation
reaction | c. | hydration reaction |
|
|
|
7.
|
a reaction involving the loss of hydrogen
|
|
|
8.
|
a reaction in which an atom or group of atoms replaces another atom or group of
atoms
|
|
|
9.
|
a reaction involving the addition of water to an alkene
|
|
|
10.
|
a reaction involving the addition of hydrogen to a carbon—carbon double
bond to produce an alkane
|
Multiple Choice (Value 18)
Identify the choice that best completes the statement or answers
the question.
|
|
|
11.
|
The IUPAC name for a carboxylic acid with two carbons in a straight chain would
be ____.
a. | ethanalic acid | c. | ethanoic acid | b. | methacarboxylic acid | d. | dimethylmethanoic
acid |
|
|
|
12.
|
Which of the following compounds will produce the least energy when completely
oxidized?
a. | hexanal | c. | hexanoic acid | b. | hexane | d. | hexanol |
|
|
|
13.
|
Which carbon skeleton contains a carboxyl group?
|
|
|
14.
|
Which of the following compounds is known as acetic acid?
|
|
|
15.
|
Aldehydes have the general structure ____________.
|
|
|
16.
|
Which carbon skeleton represents a ketone?
|
|
|
17.
|
Esters contribute which property to fruits?
a. | texture | c. | odor | b. | color | d. | skin thickness |
|
|
|
18.
|
Based on your knowledge of intermolecular forces, which of the following would
you expect to have the highest boiling point?
a. | hexanone | c. | hexanol | b. | hexane | d. | hexanal |
|
|
|
19.
|
Which of the following carbon skeletons represents a carboxylic acid?
|
|
|
20.
|
What is the name of the following compound? 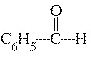 a. | benzaldehyde | c. | phenylhyde | b. | cyclohexylhyde | d. | phenol aldehyde |
|
|
|
21.
|
Which of the following compounds has the highest boiling point?
a. | 2-pentanone | c. | pentane | b. | chloropentane | d. | pentene |
|
|
|
22.
|
What is the name of the following compound? 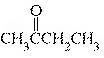 a. | butanone | c. | butanal | b. | butanol | d. | butane |
|
|
|
23.
|
What is the name of the following compound? 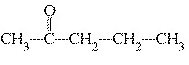 a. | 4-butanone | c. | 4-pentanone | b. | 2-butanone | d. | 2-pentanone |
|
|
|
24.
|
What happens in a condensation reaction?
a. | cross-linking of monomers | b. | head-to-tail joining of
monomers | c. | side-by-side joining of monomers | d. | substitution of a halogen on
monomers |
|
|
|
25.
|
Which of the following compounds is the most soluble in water?
a. | propanoic acid | c. | propanal | b. | propane | d. | l-bromopropane |
|
|
|
26.
|
A ketone has the general structure ____________.
|
|
|
27.
|
What type of compound is the following? 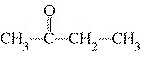 a. | alcohol | c. | ketone | b. | ether | d. | aldehyde |
|
|
|
28.
|
Which carbon skeleton represents an aldehyde?
a. |
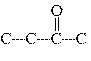 | c. |
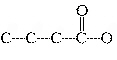 | b. |
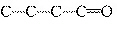
| d. |
none of
the above |
|
Short Answer: Complete 3 of the 4 below questions. (Value 6)
|
|
|
29.
|
Write the general structure for aldehyde compounds.
|
|
|
30.
|
Write the general structure for carboxylic acid compounds.
|
|
|
31.
|
Draw the structure of benzaldehyde.
|
|
|
32.
|
What is the expected product when the following compound is oxidized? CH 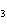 ¾ ¾CH 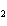 ¾ ¾CH 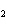 ¾ ¾CH 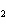 OH
|
Essay: (Value 6)
|
|
|
33.
|
Describe a polymerization condensation reaction. Give an example.
|
|
|
34.
|
Compare the properties of carboxylic acids with the properties of compounds with
other functional groups.
|