Matching (Value 4)
|
|
|
Match each item with the correct statement below. a. | substitution reaction | c. | hydration reaction | b. | addition reaction | d. | hydrogenation
reaction |
|
|
|
1.
|
a reaction in which an atom or group of atoms replaces another atom or group of
atoms
|
|
|
2.
|
a reaction in which a substance is added at the double or triple bond of an
alkene or alkyne
|
|
|
3.
|
a reaction involving the addition of water to an alkene
|
|
|
4.
|
a reaction involving the addition of hydrogen to a carbon—carbon double
bond to produce an alkane
|
Multiple Choice (Value 13)
Identify the choice that best completes the statement or answers
the question.
|
|
|
5.
|
What is the common name of the following alcohol? 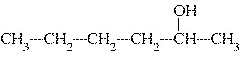 a. | tert-hexyl alcohol | c. | sec-hexyl
alcohol | b. | isohexyl alcohol | d. | hexyl alcohol |
|
|
|
6.
|
Which of the following compounds is a glycol?
|
|
|
7.
|
Which carbon skeleton represents an ether?
a. |
C¾C¾C¾O¾C¾C¾C | c. |
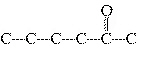 | b. |
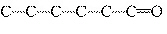 | d. |
none of the
above |
|
|
|
8.
|
What is the common name of the following compound? 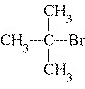 a. | sec-butyl bromide | c. | tert-butyl
bromide | b. | isopropyl bromide | d. | isobutyl bromide |
|
|
|
9.
|
What is the name of the functional group in the following compound? 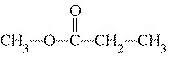 a. | carbonyl | c. | carboxylic acid | b. | halogen | d. | ester |
|
|
|
10.
|
What type of compound is CH 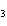 ¾ ¾O ¾CH 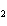 ¾ ¾CH 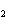 ¾ ¾CH 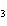 ? a. | ether | c. | alcohol | b. | aldehyde | d. | ketone |
|
|
|
11.
|
What is the carbon skeleton of the product formed in the following reaction?
C 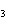 H 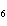 + HBr ® a. |
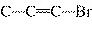 | c. |
C¾C¾C¾Br | b. |
C¾C¾Br¾C | d. |
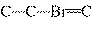 |
|
|
|
12.
|
Which halocarbon has the highest boiling point?
a. | 1,2,3-trichloropropane | c. | 2-dichloropropane | b. | 1-chloropropane | d. | 2-chloropropane |
|
|
|
13.
|
Which of the following alcohols is used in antifreeze?
a. | ethanol | c. | glycerol | b. | ethylene glycol | d. | isopropyl
alcohol |
|
|
|
14.
|
Which pair of formulas represents the same compound?
|
|
|
15.
|
Which of these compounds would you expect to be most soluble in water?
|
|
|
16.
|
Which of the following is true about isopropyl alcohol?
a. | It has a relatively high boiling point. | c. | It is completely
odorless. | b. | It is white. | d. | It is insoluble in water. |
|
|
|
17.
|
Which of the following compounds is a secondary alcohol?
a. |
CH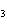 ¾CH ¾CH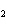 ¾CH ¾CH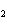 ¾CH ¾CH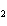 OH OH | c. |
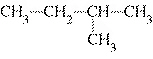 | b. |
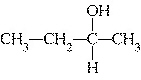 | d. |
none of the
above |
|
Short Answer (Value 2)
|
|
|
18.
|
Write an equation using structural formulas for the reaction of benzene and
chlorine.
|
|
|
19.
|
Write the general structure for halocarbon compounds.
|
Essay (Value 3)
|
|
|
20.
|
Describe what happens in a substitution reaction. Give an example of a
substitution reaction and name the atoms involved in the replacement.
|