Matching (Value 5)
|
|
|
Match each item with the correct statement below. a. | functional group | f. | halogen | b. | hydroxyl group | g. | fatty acids | c. | carbonyl
group | h. | alcohol | d. | carboxyl group | i. | glycerol | e. | ether
|
|
|
|
1.
|
a specific arrangement of atoms in an organic compound that is capable of
characteristic chemical reactions
|
|
|
2.
|
reacts with an alkane by a substitution reaction
|
|
|
3.
|
a carbon atom and an oxygen atom joined by a double bond
|
|
|
4.
|
a carbonyl group attached to a hydroxyl group
|
|
|
5.
|
carboxylic acids with long hydrocarbon chains
|
Multiple Choice (Value 15)
Identify the choice that best completes the statement or answers
the question.
|
|
|
6.
|
Which halocarbon has the highest boiling point?
a. | 1-chloropropane | c. | 1,2,3-trichloropropane | b. | 2-chloropropane | d. | 2-dichloropropane |
|
|
|
7.
|
Which of the following compounds is a secondary alcohol?
a. |
CH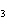 ¾CH ¾CH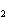 ¾CH ¾CH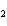 ¾CH ¾CH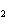 OH OH | c. |
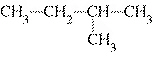 | b. |
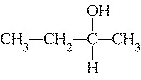 | d. |
none of the above |
|
|
|
8.
|
Which of the following alcohols is used in antifreeze?
a. | ethanol | c. | ethylene glycol | b. | isopropyl alcohol | d. | glycerol |
|
|
|
9.
|
In an addition reaction, which bond of the reactant is broken?
a. | carbon—carbon single bond | c. | carbon—carbon double
bond | b. | carbon—hydrogen single bond | d. | carbon—hydrogen double
bond |
|
|
|
10.
|
Which of the following compounds has the lowest boiling point?
a. | diethyl ether | c. | diphenyl ether | b. | 2-butanol | d. | 4-octanol |
|
|
|
11.
|
Name the following compound. CH 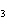 ¾ ¾CH 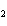 ¾ ¾CH 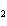 ¾ ¾CH 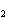 ¾ ¾O ¾C 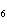 H 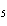 a. | cyclohexylbutyl ether | c. | phenylbutyl ether | b. | butylcyclohexyl ether | d. | butylphenyl
ether |
|
|
|
12.
|
What is the name of the following compound? 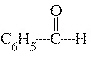 a. | phenylhyde | c. | benzaldehyde | b. | cyclohexylhyde | d. | phenol aldehyde |
|
|
|
13.
|
What is the name of the following compound? 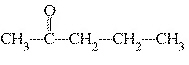 a. | 2-butanone | c. | 4-butanone | b. | 2-pentanone | d. | 4-pentanone |
|
|
|
14.
|
Which carbon skeleton represents a ketone?
|
|
|
15.
|
A ketone has the general structure ____________.
|
|
|
16.
|
Which carbon skeleton contains a carboxyl group?
|
|
|
17.
|
Which of the following carbon skeletons represents a carboxylic acid?
|
|
|
18.
|
Which of the following compounds is the most soluble in water?
a. | propanal | c. | propane | b. | l-bromopropane | d. | propanoic acid |
|
|
|
19.
|
Esters contribute which property to fruits?
a. | odor | c. | texture | b. | color | d. | skin thickness |
|
|
|
20.
|
Which of the following is a test for aldehydes?
a. | Fehling's test | c. | Butler's test | b. | flame test | d. | acid test |
|
Short Answer (Value 3)
|
|
|
21.
|
Write the general structure for halocarbon compounds.
|
|
|
22.
|
Write complete, balanced equations for the reaction of 2-pentene and water. Use
structural formulas.
|
|
|
23.
|
Write the general structure for ester compounds.
|
Essay (Value 4)
|
|
|
24.
|
Compare the properties of the alcohols with the properties of the halocarbons
and the alkanes.
|
|
|
25.
|
Describe oxidation-reduction reactions of organic molecules. Give an
example.
|