Matching (Value 5)
|
|
|
Match each item with the correct statement below. a. | condensed structural formula | d. | saturated
compound | b. | homologous series | e. | complete structural formula | c. | unsaturated
compound |
|
|
|
1.
|
group of compounds in which there is a constant increment of change in
molecular structure from one compound in the series to the next
|
|
|
2.
|
organic compound that contains at least one double or triple carbon-carbon
bond
|
|
|
3.
|
structural formula in which some bonds and/or atoms are left out
|
|
|
4.
|
formula showing all the atoms and bonds in a molecule
|
|
|
5.
|
organic compound that contains the maximum number of hydrogens per carbon
atom
|
Multiple Choice (Value 21)
Identify the choice that best completes the statement or answers
the question.
|
|
|
6.
|
What is the general formula for a straight-chain alkane?
|
|
|
7.
|
What is the name of the compound CH 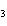 CH(CH 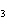 )C(CH 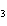 ) 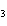 ? a. | 1,1,1,2-tetramethylpropane | c. | tetramethylpropane | b. | 2,2,3-trimethylbutane | d. | isoheptane |
|
|
|
8.
|
How many covalent bonds can each carbon atom form?
|
|
|
9.
|
What is the condensed structural formula for 2,2-dimethylbutane?
|
|
|
10.
|
How many double covalent bonds are in an alkane?
|
|
|
11.
|
What is the increment of change in a series of straight-chain alkanes?
|
|
|
12.
|
Why are the molecules of hydrocarbons nonpolar?
a. | The electron pair is shared almost equally in all the bonds. | b. | The intermolecular
attractions are strong. | c. | All the bonds are single covalent
bonds. | d. | Van der Waals forces overcome polarity. |
|
|
|
13.
|
An organic compound that contains only carbon and hydrogen and at least one
carbon-carbon triple bond is classified as an ____.
a. | alkane | c. | arene | b. | alkene | d. | alkyne |
|
|
|
14.
|
What is the simplest alkane?
a. | pentane | c. | ethane | b. | methane | d. | butane |
|
|
|
15.
|
The condensed structural formula for 2,2,3-trimethylbutane is ____.
|
|
|
16.
|
How are hydrogen atoms arranged in ethene?
a. | in the same plane, separated by angles of 180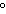 | b. | in different planes, separated by angles of 120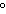 | c. | in different planes, separated by angles of 180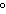 | d. | in the same plane, separated by angles of 120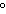 |
|
|
|
17.
|
Which of these compounds is an alkene?
a. | nonene | c. | methane | b. | butyne | d. | propanone |
|
|
|
18.
|
How many valence electrons does a carbon atom have?
|
|
|
19.
|
What is the physical state of the smallest alkanes at room temperature?
a. | gas | c. | liquid | b. | gas or liquid | d. | solid |
|
|
|
20.
|
Which of the following compounds is an unsaturated hydrocarbon?
a. | methyl | c. | propyne | b. | nonane | d. | methane |
|
|
|
21.
|
What is the name of the alkane having five carbons?
a. | pentane | c. | octane | b. | propane | d. | methane |
|
|
|
22.
|
How many carbons are in a molecule of hexane?
|
|
|
23.
|
What is the name of the smallest alkyne?
a. | ethyne | c. | methyne | b. | butyne | d. | propyne |
|
|
|
24.
|
A saturated straight-chain hydrocarbon with two carbons is ____.
a. | decane | c. | ethane | b. | propane | d. | ethene |
|
|
|
25.
|
The name for an alkyl group that contains two carbon atoms is ____.
a. | diphenyl | c. | dimethyl | b. | propyl | d. | ethyl |
|
|
|
26.
|
In which of the following compounds does rotation occur around all covalent
bonds between carbons?
a. | octene | c. | octane | b. | octyne | d. | all of the
above |
|