Multiple Choice (Value 15) Two Bonus Questions
Identify the choice that best completes the statement or answers
the question.
|
|
|
1.
|
Which of the following statements explains why the melting of ice is a
spontaneous reaction at room temperature and pressure?
a. | Melting is accompanied by a decrease of entropy. | b. | Melting is
accompanied by an increase of energy. | c. | Melting is accompanied by an increase of
entropy. | d. | Melting is accompanied by a decrease of energy. |
|
|
|
2.
|
In which of these systems is the entropy decreasing?
a. | a liquid cooling | c. | salt dissolving in water | b. | snow
melting | d. | air escaping from a
tire |
|
|
|
3.
|
The melting of ice at temperatures above 0 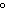 C ____. a. | is endothermic | c. | liberates heat | b. | is not favorable | d. | is not
spontaneous |
|
|
|
4.
|
If a system is left to change spontaneously, in what state will it end?
a. | the state with lowest possible energy | b. | the same state in which it
began | c. | the state with the maximum disorder | d. | the state with the lowest possible energy
consistent with the state of maximum disorder |
|
|
|
5.
|
The amount of disorder in a system is measured by its ____.
a. | entropy | c. | K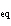 | b. | equilibrium
position | d. | activation
energy |
|
|
|
6.
|
Which physical state of nitrogen has the highest entropy?
a. | solid | c. | liquid | b. | gas | d. | vapor |
|
|
|
7.
|
The two factors that determine whether a reaction is spontaneous or
nonspontaneous are ____.
a. | energy and heat of reaction | b. | entropy and energy | c. | entropy and
disorder | d. | electron configuration and ionic charge |
|
|
|
8.
|
Which of the following is true about the numerical value of Gibbs free-energy
change for a spontaneous reaction?
a. | It is not related to enthalpy. | b. | It is negative. | c. | It is positive for
temperatures above 850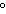 C. C. | d. | It indicates that work must be
expended. |
|
|
|
9.
|
Spontaneous reactions ____.
a. | always take place at a rapid rate | b. | always result in increased disorder of the
system | c. | are always exothermic | d. | always release free
energy |
|
|
|
10.
|
Entropy measures ____.
a. | energy | c. | disorder | b. | force | d. | heat
transferred |
|
|
|
11.
|
Which one of the following systems has the highest entropy?
a. | 10 mL of water at 100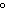 C C | b. | 10 mL of water at 10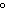 C C | c. | 10 mL of water at 50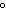 C C | d. | All have the same entropy because all are
water. |
|
|
|
12.
|
The energy that is available to do work in a reaction is called ____.
a. | free energy | c. | entropy | b. | enthalpy | d. | heat |
|
|
|
13.
|
Which reaction results in the greatest increase in entropy?
a. | A ® B | c. | 2A ® B | b. | A ®
2B | d. | 3A ® B |
|
|
|
14.
|
Which of the following is true about the combustion of carbon?
a. | Entropy decreases. | b. | The reaction is
spontaneous. | c. | Enthalpy remains constant. | d. | Carbon is produced from oxygen and carbon
dioxide. |
|
|
|
15.
|
What determines whether or not a reaction is spontaneous?
a. | enthalpy change and entropy change | b. | change in molar volume and heat
change | c. | change in enthalpy only | d. | change in entropy
only |
|
Short Answer: Complete one the three below questions (Value 3)
|
|
|
16.
|
Calculate the concentration of a silver ion when the solubility product constant
of AgI is 10 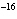 .
|