Matching
|
|
|
Match each item with the correct statement below. a. | calorimeter | d. | enthalpy | b. | calorie | e. | specific heat | c. | joule | f. | heat
capacity |
|
|
|
1.
|
quantity of heat needed to raise the temperature of 1 g of water by 1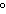 C C
|
|
|
2.
|
SI unit of energy
|
|
|
3.
|
quantity of heat needed to change the temperature of 1 g of a substance by
1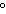 C C
|
|
|
4.
|
quantity of heat needed to change the temperature of an object by 1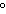 C C
|
|
|
5.
|
device used to measure the heat absorbed or released during a chemical or
physical process
|
|
|
6.
|
heat content of a system at constant pressure
|
|
|
Match each item with the correct statement below. a. | heat of reaction | d. | heat of fusion | b. | heat of formation | e. | heat of solution | c. | Hess's law of
heat summation |
|
|
|
7.
|
the enthalpy change for a chemical reaction exactly as it is written
|
|
|
8.
|
the enthalpy change caused by dissolving a substance
|
|
|
9.
|
the energy required to melt a solid at its melting point
|
|
|
10.
|
the change in enthalpy that accompanies the formation of a compound from its
elements
|
|
|
11.
|
states that if you add two or more thermochemical equations to give a final
equation, you can also add the heats of reaction to give the final heat of reaction
|
Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
12.
|
A piece of metal is heated, then submerged in cool water. Which statement below
describes what happens?
a. | The temperature of the metal will increase. | b. | The temperature of
the water will increase. | c. | The temperature of the water will
decrease. | d. | The temperature of the water will increase and the temperature of the metal will
decrease. |
|
|
|
13.
|
Which of the following is NOT a form of energy?
a. | light | c. | heat | b. | pressure | d. | electricity |
|
|
|
14.
|
When energy is changed from one form to another, ____.
a. | some of the energy is lost entirely | b. | all of the energy can be accounted
for | c. | a physical change occurs | d. | all of the energy is changed to a useful
form |
|
|
|
15.
|
When your body breaks down sugar completely, how much heat is released compared
to burning the same amount of sugar in a flame?
a. | The body releases more heat. | b. | The body releases less
heat. | c. | The body releases the same amount of heat. | d. | The body releases no
heat. |
|
|
|
16.
|
The quantity of heat required to change the temperature of 1 g of a substance by
1 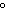 C is defined as ____. a. | a joule | c. | a calorie | b. | specific heat | d. | density |
|
|
|
17.
|
A piece of candy has 5 Calories (or 5000 calories). If it could be burned,
leaving nothing but carbon dioxide and water, how much heat would it give off?
a. | 500 calories | c. | 5000 joules | b. | 5 kilocalories | d. | Not enough information is
given. |
|
|
|
18.
|
How many joules are in 148 calories? (1 cal = 4.18 J)
a. | 6.61 J | c. | 148 J | b. | 35.4 J | d. | 619 J |
|
|
|
19.
|
Which of the following is a valid unit for specific heat?
|
|
|
20.
|
When 45 g of an alloy, at 25 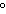 C, are dropped into 100.0 g of
water, the alloy absorbs 956 J of heat. If the final temperature of the alloy is 37 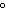 C,
what is its specific heat? a. | 0.423 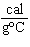 | c. | 9.88 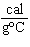 | b. | 1.77 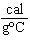 | d. | 48.8 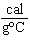 |
|
|
|
21.
|
Which of the following has the greatest heat capacity?
a. | 1000 g of water | c. | 1 g of water | b. | 1000 g of steel | d. | 1 g of steel |
|
|
|
22.
|
By what quantity must the heat capacity of an object be divided to obtain the
specific heat of that material?
a. | its mass | c. | its temperature | b. | its volume | d. | its energy |
|
|
|
23.
|
Standard conditions of temperature and pressure for a thermochemical equation
are ____.
a. | 0 C and 101 kPa C and 101 kPa | c. | 0 C and 0
kPa C and 0
kPa | b. | 25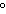 C and 101 kPa C and 101 kPa | d. | 25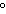 C and 22.4
kPa C and 22.4
kPa |
|
|
|
24.
|
The heat content of a system is equal to the enthalpy only for a system that is
at constant ____.
a. | temperature | c. | pressure | b. | volume | d. | mass |
|
|
|
25.
|
On what principle does calorimetry depend?
a. | Hess's law | c. | law of enthalpy | b. | law of conservation of
energy | d. | law of multiple
proportions |
|
|
|
26.
|
How can the enthalpy change be determined for a reaction in an aqueous
solution?
a. | by knowing the specific heat of the reactants | b. | by mixing the
reactants in a calorimeter and measuring the temperature change | c. | by knowing the mass
of the reactants | d. | The enthalpy change for this type of reaction cannot be
determined. |
|
|
|
27.
|
A chunk of ice whose temperature is –20 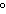 C is added to
an insulated cup filled with water at 0 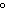 C. What happens in the cup? a. | The ice melts until it reaches the temperature of the water. | b. | The water cools
until it reaches the temperature of the ice. | c. | Some of the water freezes, so the chunk of ice
gets larger. | d. | none of the above |
|
|
|
28.
|
Calculate the energy required to produce 7.00 mol Cl 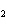 O 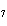 on
the basis of the following balanced equation. 2Cl 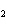 ( g) + 7O 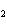 ( g) + 130 kcal 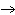 2Cl 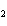 O 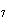 ( g) a. | 7.00 kcal | c. | 130 kcal | b. | 65 kcal | d. | 455 kcal |
|
|
|
29.
|
The amount of heat needed to melt one mole of a solid at a constant temperature
is called ____.
a. | molar heat of fusion | c. | heat of reaction | b. | molar heat of
solidification | d. | enthalpy |
|
|
|
30.
|
What is the heat of solution?
a. | the amount of heat required to change a solid into a liquid | b. | the amount of heat
absorbed or released when a solid dissolves | c. | the amount of heat required to change a vapor
into a liquid | d. | the amount of heat released when a vapor changes into a
liquid |
|
|
|
31.
|
When 10 g of diethyl ether is converted to vapor at its boiling point, about how
much heat is absorbed? (C 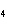 H 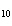 O,  H H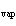 = 15.7 kJ/mol, boiling point: 34.6 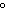 C) a. | 2 kJ | c. | 0.2 kJ | b. | 2 J | d. | Not enough information is
given. |
|
|
|
32.
|
Hess's law ____.
a. | makes it possible to calculate  H for complicated
chemical reactions H for complicated
chemical reactions | b. | states that when you reverse a chemical
equation, you must change the sign of  H H | c. | determines the way a
calorimeter works | d. | describes the vaporization of
solids |
|
|
|
33.
|
The amount of heat involved in the synthesis of 1 mole of a compound from its
elements, with all substances in their standard states at 25 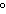 C, is called ____. a. | enthalpy | c. | standard heat of formation | b. | heat of
reaction | d. | heat of
solidification |
|
|
|
34.
|
 H H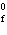 for the formation of rust
(Fe 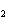 O 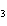 ) is –826 kJ/mol. How
much energy is involved in the formation of 5 grams of rust? a. | 25.9 kJ | c. | 66 kJ | b. | 25.9 J | d. | 66 J |
|
|
|
35.
|
Calculate  H H for the reaction of sulfur dioxide with
oxygen. 2SO 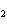 ( g) + O 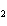 ( g)
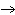 2SO 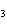 ( g) (  H H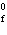 SO 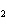 ( g) = –296.8 kJ/mol;  H H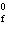 SO 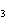 ( g) = –395.7 kJ/mol) a. | –98.9 kJ | c. | 197.8 kJ | b. | –197.8 kJ | d. | Not enough information is
given. |
|